Abstract
Background
Acute exercise can increase skeletal muscle citrate synthase (CS) enzyme activity and resting skeletal muscle mitochondrial enzyme activity has been linked to maximal oxygen consumption (\(\dot {V}{{\text{O}}_{2\hbox{max} }}\)). We investigated: (1) if acute aerobic exercise (AEx) increases muscle metabolic enzyme activities other than CS; (2) if the addition of acute resistance exercise (REx) enhances the response to AEx (A + REx); and (3) if post-exercise muscle metabolic enzyme activity was related to \(\dot {V}{{\text{O}}_{2\hbox{max} }}\).
Methods
Twelve young, sedentary men completed 45 min of two-legged cycle ergometry at 55% of \(\dot {V}{{\text{O}}_{2\hbox{max} }}\) and 3 sets of 8–12 repetitions of one-leg knee extensor at 55% of 1 repetition maximum (1-RM). Vastus lateralis biopsies were taken prior to and 1 h post AEx and A + REx for the measurement of phosphofructokinase (PFK), 3-l-hydroxyacyl CoA dehydrogenase (β-HAD), succinate dehydrogenase (SDH) and CS.
Results
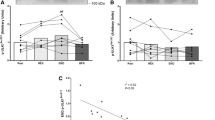
As a group, there was no effect of acute AEx or A + REx on muscle PFK, β-HAD, CS, and SDH activities. Post exercise muscle PFK, β-HAD, CS, and SDH activities were related to higher \(\dot {V}{{\text{O}}_{2\hbox{max} }}\) (r = 0.62–0.74). With participants grouped by \(\dot {V}{{\text{O}}_{2\hbox{max} }}\) (LOW, < 30th %; NORM, > 50th %), acute exercise-induced changes in muscle PFK, β-HAD, CS, and SDH were greater in NORM compared to LOW.
Conclusion
These findings suggest acute exercise muscle metabolic enzyme activities are predictive of \(\dot {V}{{\text{O}}_{2\hbox{max} }}\) and possibly supportive of higher \(\dot {V}{{\text{O}}_{2\hbox{max} }}\). Also, low \(\dot {V}{{\text{O}}_{2\hbox{max} }}\) (below 30th percentile) appears to impair skeletal muscle metabolic enzyme responses to acute exercise.




Similar content being viewed by others
Abbreviations
- AEx:
-
Aerobic exercise
- A + REx:
-
Aerobic + resistance exercise
- β-HAD:
-
3-l-Hydroxyacyl CoA dehydrogenase
- BMI:
-
Body mass index
- CS:
-
Citrate synthase
- FPG:
-
Fasting plasma glucose
- HDL:
-
High density lipoprotein
- HOMA-β:
-
Homeostasis model assessment-β cell function
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance
- KE:
-
Maximal knee extensor strength
- LDL:
-
Low-density lipoprotein
- PFK:
-
Phosphofructokinase
- PRE:
-
Prior to exercise
- SDH:
-
Succinate dehydrogenase
- TC:
-
Total cholesterol
- \(\dot {V}{{\text{O}}_{2\hbox{max} }}\) :
-
Maximal oxygen consumption
References
Henriksson J, Reitman JS (1976) Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand 97(3):392–397. https://doi.org/10.1111/j.1748-1716.1976.tb10279.x
Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC, Bouchard C (1987) Effects of two high-intensity intermittent training programs interspaced by detraining on human skeletal muscle and performance. Eur J Appl Physiol Occup Physiol 56(5):516–521
Tang JE, Hartman JW, Phillips SM (2006) Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab 31(5):495–501. https://doi.org/10.1139/h06-026
Komi PV, Viitasalo JT, Rauramaa R, Vihko V (1978) Effect of isometric strength training of mechanical, electrical, and metabolic aspects of muscle function. Eur J Appl Physiol Occup Physiol 40(1):45–55
Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS (2001) Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280(2):R441–R447
Tonkonogi M, Harris B, Sahlin K (1997) Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand 161(3):435–436. https://doi.org/10.1046/j.1365-201X.1997.00233.x
Dohm GL, Huston RL, Askew EW, Fleshood HL (1973) Effects of exercise, training, and diet on muscle citric acid cycle enzyme activity. Can J Biochem 51(6):849–854
Ji LL, Stratman FW, Lardy HA (1988) Enzymatic down regulation with exercise in rat skeletal muscle. Arch Biochem Biophys 263(1):137–149
Lawler JM, Powers SK, Visser T, Van Dijk H, Kordus MJ, Ji LL (1993) Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fiber type and age. Am J Physiol 265(6 Pt 2):R1344–R1350. https://doi.org/10.1152/ajpregu.1993.265.6.R1344
Gollnick PD, Bertocci LA, Kelso TB, Witt EH, Hodgson DR (1990) The effect of high-intensity exercise on the respiratory capacity of skeletal muscle. Pflugers Arch 415(4):407–413
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports M (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Sale DG, MacDougall JD, Jacobs I, Garner S (1990) Interaction between concurrent strength and endurance training. J Appl Physiol (1985) 68(1):260–270
Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J (2007) Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 147(6):357–369
Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP (2010) Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 304(20):2253–2262. https://doi.org/10.1001/jama.2010.1710
Sparks LM, Johannsen NM, Church TS, Earnest CP, Moonen-Kornips E, Moro C, Hesselink MK, Smith SR, Schrauwen P (2013) Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab 98(4):1694–1702. https://doi.org/10.1210/jc.2012-3874
Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, Yamazaki T, Froelicher V (2004) Fitness versus physical activity patterns in predicting mortality in men. Am J Med 117(12):912–918. https://doi.org/10.1016/j.amjmed.2004.06.047
Gavin TP, Van Meter JB, Brophy PM, Dubis GS, Potts KN, Hickner RC (2012) Comparison of a field-based test to estimate functional threshold power and power output at lactate threshold. J Strength Cond Res 26(2):416–421. https://doi.org/10.1519/JSC.0b013e318220b4eb
Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA (1997) Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82(5):1573–1583
Hoppeler H (1990) The different relationship of VO2max to muscle mitochondria in humans and quadrupedal animals. Respir Physiol 80(2–3):137–145
Goodpaster BH, Sparks LM (2017) Metabolic flexibility in health and disease. Cell Metab 25(5):1027–1036. https://doi.org/10.1016/j.cmet.2017.04.015
Goodpaster BH, Wolfe RR, Kelley DE (2002) Effects of obesity on substrate utilization during exercise. Obes Res 10(7):575–584. https://doi.org/10.1038/oby.2002.78
Prior SJ, Ryan AS, Stevenson TG, Goldberg AP (2014) Metabolic inflexibility during submaximal aerobic exercise is associated with glucose intolerance in obese older adults. Obesity (Silver Spring) 22(2):451–457. https://doi.org/10.1002/oby.20609
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30(12):1000–1007
Gavin TP, Stallings HW III, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC (2005) Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol 98(1):315–321
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7(6):1235–1246. https://doi.org/10.1038/nprot.2012.058
Shan C, Elf S, Ji Q, Kang HB, Zhou L, Hitosugi T, Jin L, Lin R, Zhang L, Seo JH, Xie J, Tucker M, Gu TL, Sudderth J, Jiang L, DeBerardinis RJ, Wu S, Li Y, Mao H, Chen PR, Wang D, Chen GZ, Lonial S, Arellano ML, Khoury HJ, Khuri FR, Lee BH, Brat DJ, Ye K, Boggon TJ, He C, Kang S, Fan J, Chen J (2014) Lysine acetylation activates 6-phosphogluconate dehydrogenase to promote tumor growth. Mol Cell 55(4):552–565. https://doi.org/10.1016/j.molcel.2014.06.020
Jaworowski A, Porter MM, Holmback AM, Downham D, Lexell J (2002) Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176(3):215–225. https://doi.org/10.1046/j.1365-201X.2002.t01-2-01004.x
Nie Y, Sato Y, Wang C, Yue F, Kuang S, Gavin TP (2016) Impaired exercise tolerance, mitochondrial biogenesis, and muscle fiber maintenance in miR-133a-deficient mice. FASEB J 30(11):3745–3758. https://doi.org/10.1096/fj.201600529R
Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP (2008) AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol 586:6021–6035
Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW (2002) Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol (1985) 92(6):2475–2482. https://doi.org/10.1152/japplphysiol.00071.2002
ACSM (2000) ACSM’s guidlelines for exercise testing and prescription, 6th edn. American College of Sports Medicine, Baltimore
Blaha MJ, Hung RK, Dardari Z, Feldman DI, Whelton SP, Nasir K, Blumenthal RS, Brawner CA, Ehrman JK, Keteyian SJ, Al-Mallah MH (2016) Age-dependent prognostic value of exercise capacity and derivation of fitness-associated biologic age. Heart. https://doi.org/10.1136/heartjnl-2015-308537
Roepstorff C, Schjerling P, Vistisen B, Madsen M, Steffensen CH, Rider MH, Kiens B (2005) Regulation of oxidative enzyme activity and eukaryotic elongation factor 2 in human skeletal muscle: influence of gender and exercise. Acta Physiol Scand 184(3):215–224. https://doi.org/10.1111/j.1365-201X.2005.01442.x
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol (1985) 75(2):712–719
Hargreaves M (2015) Exercise, muscle, and CHO metabolism. Scand J Med Sci Sports 25(Suppl 4):29–33. https://doi.org/10.1111/sms.12607
Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stockli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA, James DE (2015) Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab 22(5):922–935. https://doi.org/10.1016/j.cmet.2015.09.001
Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. https://doi.org/10.1042/bse0470069
Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA (2002) Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51(2):284–292
Ferretti G (2014) Maximal oxygen consumption in healthy humans: theories and facts. Eur J Appl Physiol 114(10):2007–2036. https://doi.org/10.1007/s00421-014-2911-0
Wagner PD (1996) Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58:21–50
Hickson RC (1980) Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol 45(2–3):255–263
Dudley GA, Djamil R (1985) Incompatibility of endurance- and strength-training modes of exercise. J Appl Physiol (1985) 59(5):1446–1451
Abbasi F, Brown BW Jr, Lamendola C, McLaughlin T, Reaven GM (2002) Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 40(5):937–943
Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr, Blair SN (1999) Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282(16):1547–1553
Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA (2002) Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 25(9):1612–1618
Koivisto VA, Yki-Jarvinen H, DeFronzo RA (1986) Physical training and insulin sensitivity. Diabetes Metab Rev 1(4):445–481
Acknowledgements
The authors wish to thank Bruno Roseguini, Mandi Kulbersh, and Alanna Fennimore for their assistance and the volunteers who took part in the study. This research project was supported by intramural funds from Purdue University.
Author information
Authors and Affiliations
Contributions
YN and JSS assisted with data collection, data analysis, contributed to the discussion, and wrote/edited the manuscript. JAW, RTG, SK, and JS assisted with data collection and analysis. TPG designed the experiment, assisted with data collection and data analysis, contributed to the discussion, and wrote/edited the manuscript. TPG is the guarantor of this work, and as such, takes responsibility for the integrity of the data and accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
TPG is on the Advisory Board for Sport Sciences for Health. YN, JSS, JAW, RTG, SK, JS, and TPG report no potential conflicts of interest relevant to this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Solfest, J.S., Nie, Y., Weiss, J.A. et al. Effects of acute aerobic and concurrent exercise on skeletal muscle metabolic enzymes in untrained men. Sport Sci Health 15, 417–426 (2019). https://doi.org/10.1007/s11332-019-00547-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-019-00547-z