Abstract
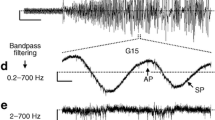
The membrane potential (V m) of beta-cells oscillates at glucose concentrations between ~6 and 25 mM, i.e. burst phases with action potentials alternate with silent interburst phases generating so-called slow waves. The slow waves drive oscillations of the cytosolic Ca2+ concentration ([Ca2+]c) and insulin secretion. The length of the bursts correlates with the amount of insulin release. Thus, the fraction of plateau phase (FOPP), i.e. the percentage of time with burst activity, is an excellent marker for beta-cell function and metabolic integrity. Extracellular voltage changes of mouse islets were measured using a microelectrode array (MEA) allowing the detection of burst and interburst phases. At a non-stimulating glucose concentration (3 mM) no electrical activity was detectable while bursting was continuous at 30 mM. The glucose concentration–response (determined as FOPP) curve revealed half-maximal stimulation at 12 ± 1 mM (Hill equation fit). The signal was sensitive to KATP channel modulators, e.g. tolbutamide or diazoxide. Simultaneous recordings of electrical activity and [Ca2+]c revealed congruent bursts and peaks, respectively. The extracellular recordings are in perfect agreement with more time-consuming intracellular electrical recordings. The results provide a 'proof-of-principle' for detection of beta-cell slow waves and determination of the FOPP using extracellular electrodes in a MEA-based system. The method is facile and provides the capability to study the effects of modulators of beta-cell function including possible anti-diabetic drugs in real time. Moreover, the method may be useful for checking the metabolic integrity of human donor islets prior to transplantation.





Similar content being viewed by others
References
Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54:87–143
Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B (1994) Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J Biol Chem 269:8749–53
Bornat Y, Raoux M, Boutaib Y, Morin F, Charpentier C, Lang J, Renaud S (2010) Detection of electrical activity of pancreatic beta-cells using micro-electrode arrays. IEEE International Symposium on Electronic Design, Test & Applications 5:233–236
Dean PM, Matthews EK (1968) Electrical activity in pancreatic islet cells. Nature 219:389–90
Dishinger JF, Reid KR, Kennedy RT (2009) Quantitative monitoring of insulin secretion from single islets of Langerhans in parallel on a microfluidic chip. Anal Chem 81:3119–27
Drews G, Krämer C, Düfer M, Krippeit-Drews P (2000) Contrasting effects of alloxan on islets and single mouse pancreatic beta-cells. Biochem J 352(Pt 2):389–97
Drews G, Krippeit-Drews P, Düfer M (2010) Electrophysiology of islet cells. Adv Exp Med Biol 654:115–63
Düfer M, Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G (2004) Oscillations of membrane potential and cytosolic Ca2+ concentration in SUR1−/− beta cells. Diabetologia 47:488–98
Gilon P, Ravier MA, Jonas JC, Henquin JC (2002) Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes 51(Suppl 1):S144–51
Gilon P, Shepherd RM, Henquin JC (1993) Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem 268:22265–8
Göpel S, Kanno T, Barg S, Galvanovskis J, Rorsman P (1999) Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol 521(Pt 3):717–28
Henquin JC (2009) Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52:739–51
Henquin JC, Meissner HP (1984) Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia 40:1043–52
Hofmann F, Bading H (2006) Long term recordings with microelectrode arrays: studies of transcription-dependent neuronal plasticity and axonal regeneration. J Physiol Paris 99:125–32
Khan FA, Goforth PB, Zhang M, Satin LS (2001) Insulin activates ATP-sensitive K+ channels in pancreatic beta-cells through a phosphatidylinositol 3-kinase-dependent pathway. Diabetes 50:2192–8
Luo L, Badiavas E, Luo JZ, Maizel A (2007) Allogeneic bone marrow supports human islet beta cell survival and function over six months. Biochem Biophys Res Commun 361:859–64
Palti Y, David GB, Lachov E, Mida YH, Schatzberger R (1996) Islets of Langerhans generate wavelike electric activity modulated by glucose concentration. Diabetes 45:595–601
Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M (1991) Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Arch - Eur J Physiol 418:417–22
Schulze DU, Düfer M, Wieringa B, Krippeit-Drews P, Drews G (2007) An adenylate kinase is involved in KATP channel regulation of mouse pancreatic beta cells. Diabetologia 50:2126–34
Stett A, Egert U, Guenther E, Hofmann F, Meyer T, Nisch W, Haemmerle H (2003) Biological application of microelectrode arrays in drug discovery and basic research. Anal Bioanal Chem 377:486–95
You S, Rivereau AS, Gouin E, Sai P (2001) Co-incubation of pig islet cells with spleen cells from non-obese diabetic mice causes decreased insulin release by non-T-cell- and T-cell-mediated mechanisms. Clin Exp Immunol 125:25–31
Acknowledgement
This work was supported by grants from the Deutsche Forschungsgemeinschaft (G.D. and M.D.). We thank Professor Joseph Bryan for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfeiffer, T., Kraushaar, U., Düfer, M. et al. Rapid functional evaluation of beta-cells by extracellular recording of membrane potential oscillations with microelectrode arrays. Pflugers Arch - Eur J Physiol 462, 835–840 (2011). https://doi.org/10.1007/s00424-011-1029-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-1029-z