Abstract
CO2 chemosensing is a vital function for the maintenance of life that helps to control acid–base balance. Most studies have reported that CO2 is measured via its proxy, pH. Here we report an inwardly rectifying channel, in outside-out excised patches from HeLa cells that was sensitive to modest changes in PCO2 under conditions of constant extracellular pH. As PCO2 increased, the open probability of the channel increased. The single-channel currents had a conductance of 6.7 pS and a reversal potential of –70 mV, which lay between the K+ and Cl– equilibrium potentials. This reversal potential was shifted by +61 mV following a tenfold increase in extracellular [K+] but was insensitive to variations of extracellular [Cl–]. The single-channel conductance increased with extracellular [K+]. We propose that this channel is a member of the Kir family. In addition to this K+ channel, we found that many of the excised patches also contained a conductance carried via a Cl–-selective channel. This CO2-sensitive Kir channel may hyperpolarize excitable cells and provides a potential mechanism for CO2-dependent inhibition during hypercapnia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regulation of the levels of blood gases (oxygen and carbon dioxide) is vitally important in the maintenance of life. Of the two gases, CO2 appears to be the more powerful stimulant, as PCO2 in arterial blood is very well controlled at a range of increasing altitudes even though atmospheric PO2 decreases [8]. Only when extreme altitudes (3,800–4,300 m) are reached or a prolonged period is spent at altitude [2] does the regulation of arterial PCO2 change. By contrast arterial PO2 varies dramatically during short-term exposure to moderate altitude; therefore, arterial PCO2 is tightly regulated at the expense of PO2 [8].
CO2 combines with water to form H2CO3. This reaction is slow and its rate can be dramatically increased by carbonic anhydrase. Once formed H2CO3 rapidly dissociates to HCO –3 and H+. Thus the level of dissolved CO2 in the extracellular fluid (ECF) determines its pH. In principle, PCO2 could be measured in three ways: via CO2 itself, via pH, or via HCO –3 . There is considerable evidence that changes in pH are important in chemoreception; however, evidences for the involvement of HCO –3 [22] and CO2 are beginning to emerge [11, 12]. Although CO2/pH-sensitive cells are located in the carotid bodies [18], the major sites of CO2 chemoreception are found within the brain [4].
To be classified as a primary CO2/pH chemosensor, a cell must have certain properties. Firstly, they need to possess a transducer molecule that responds to alterations of CO2, HCO –3 or pH. Secondly, they have to project to areas responsible for initiating chemoreflexes and finally, once stimulated they must initiate a physiological response [7, 20, 21]. K+ channels that are highly sensitive to acidification and react by closing are popular candidates for chemosensory transducers. These include the following: TASK (tandem-pore acid sensing potassium channels) 1 and 3 channels [17]; inwardly rectifying potassium channels [19], especially Kir4.1/5.1, and calcium-dependent potassium channels [5]. However, definitive causal evidence linking these channels to behavioral/physiological responses to changes in PCO2 has not yet been achieved. Acid-sensitive cation channels (ASICs) also play a role in at least some CO2-dependent processes [34].
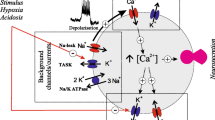
By contrast, there are very few examples of, or putative mechanisms for, direct sensing of CO2 [11, 12, 35]. Here we describe a K+ channel in HeLa cells that appears to open in a CO2-sensitive manner. If present in neurons this channel would mediate CO2-dependent hyperpolarization and decreased neuronal firing. Though less attention has been paid to inhibitory processes triggered by increases in PCO2, neurons that are inhibited by CO2 may be as important as those excited by it [20]. Alternatively, in peripheral tissue this could lead to increased secretion from acinar cells of the major glands.
Methods
Cell culture
HeLa cells (either wild type or Cx26 expressing) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with the following supplements: 1 mM glutamine (Melford Labs, Suffolk, UK), 10% foetal calf serum (Invitrogen, PaisleyUK), and penicillin/streptomycin (Sigma, St. Louis, MO, USA) at 10 U/ml and 10 μg respectively. In addition, the Cx26 cells were under selective pressure with puromycin (Sigma) at 1 μg/ml. All cells were grown at 37°C in a humidified 95% O2/5% CO2 incubator. For patch clamp recordings, the cells were plated out on glass coverslips in six-well plates at 2 × 106 cells/well and used within 3 days from plating.
Patch clamp recordings
Coverslips containing non-confluent cells were placed into a perfusion chamber at 28°C in sterile filtered control artificial cerebrospinal fluid (aCSF): Standard patch clamp techniques were used to pull outside-out isolated membrane patches. Whole cell patch pipettes were pulled on a Flaming–Brown horizontal puller, Sylgard coated, fire polished, and filled with an intracellular solution: K-gluconate 120 mM, CsCl2 10 mM, TEACl 10 mM, EGTA 10 mM, ATP 3 mM, MgCl2 1 mM, CaCl2 1 mM, sterile filtered, pH adjusted to 7.2 with KOH. After briefly attaining a whole cell recording, isolated patches of the outside-out configuration were excised. To examine the effect of PCO2 on channel gating, patches were routinely held at +10 mV. The properties of the CO2-sensitive single-channel current were assessed by taking the patch through a series of 10 mV steps from either +20 or +50 mV to –70 mV in aCSF with a PCO2 of 70 mmHg.
An Axopatch 200B amplifier was used (usually in capacitive feedback mode) to record from the membrane patches. The data were low-pass filtered by the amplifier with a cut-off of 2 kHz. The data were sampled by a DT3010 A/D board at 20 kHz. Proprietary software was used to control the experiments and perform offline analysis. For analysis and measurements, the current records were filtered with a Gaussian filter at 0.5 or 0.8 kHz. The reset transients from the feedback capacitor were excluded from analysis (by choosing portions of current records between these transients) and removed from the illustrations in the paper.
Recording solutions
Control aCSF
124 mM NaCl, 3 mM KCl, 1 mM CaCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, 1 mM MgSO4, 10 mM d-glucose saturated with 95% O2/5% CO2, pH 7.5, PCO2 35 mmHg.
PCO2 70 mmHg aCSF
70 mM NaCl, 3 mM KCl, 1 mM CaCl2, 80 mM NaHCO3, 1.25 mM NaH2PO4, 1 mM MgSO4, 10 mM d-glucose, saturated with approximately 12% CO2 (with the balance being O2) to give a pH of 7.5 and a PCO2 of 70 mmHg.
PCO2 55 mmHg aCSF
100 mM NaCl, 3 mM KCl, 1 mM CaCl2, 50 mM NaHCO3, 1.25 mM NaH2PO4, 1 mM MgSO4, 10 mM d-glucose, saturated with approximately 9% CO2 (with the balance being O2) to give a pH of 7.5 and a PCO2 of 55 mmHg respectively.
PCO2 20 mmHg aCSF
140 mM NaCl, 3 mM KCl, 1 mM CaCl2, 10 mM NaHCO3, 1.25 mM NaH2PO4, 1 mM MgSO4, 10 mM d-glucose, saturated with approximately 2% CO2 (with the balance being O2) to give a pH of 7.5 and a PCO2 of 20 mmHg.
The level of PCO2 was adjusted (by varying the proportion of CO2 in the bubbling mixture) so that all solutions had a pH of 7.5. The level of PCO2 in these solutions was determined by measurement with a blood gas analyzer [12].
Ion substitution experiments
To test the nature of the permeant ion, we substituted either Cl– or K+. For Cl– substitution, we used a modified aCSF with Na-gluconate: 10 mM NaCl, 114 mM Na-gluconate, 26 mM NaHCO3, 1.25 mM NaH2PO4, 3 mM KCl, 1 mM MgSO4, 1 mM CaCl2, and 10 mM d-glucose, equilibrated with 5% CO2/95% O2.
The patches were also exposed to an isohydric hypercapnic stimulus under conditions of lowered Cl– concentrations: 10 mM NaCl, 70 mM Na-gluconate, 80 mM NaHCO3, 1.25 mM NaH2PO4, 3 mM KCl, 1 mM MgSO4, 1 mM CaCl2, and 10 mM d-glucose, equilibrated with 12% CO2/88% O2.
To change K+, we used a modified aCSF of the following composition: 97 mM NaCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 30 mM KCl, 1 mM MgSO4, 1 mM CaCl2, and 10 mM d-glucose, equilibrated with 95%O2, 5% CO2. Patches were exposed to an isohydric hypercapnic solution with elevated K+ concentrations: 43 mM NaCl, 80 mM NaHCO3, 1.25 mM NaH2PO4, 30 mM KCl, 1 mM MgSO4, 1 mM CaCl2, and 10 mM d-glucose, equilibrated with 12% CO2/88% O2.
Data analysis and statistics
To analyze how the single-channel current varied with transmembrane voltage and ionic conditions, a sum of Gaussian distributions was fitted (by a least-squares method) to an all-points histogram of channel activity at each transmembrane potential. The current amplitude of the unitary single-channel openings was estimated from these fits (mean of single-channel current minus mean of the noise). The single-channel conductance and reversal potentials were estimated by fitting lines to the linear portion of the current–voltage relation. Expected equilibrium potentials were calculated from the Nernst equation based on the composition of the solutions used in the patch pipette and bathing medium.
To analyze the effect of PCO2 on channel gating, the open probability of the channel was estimated from all-points histograms. The area under the curve corresponding to single-channel or multiple-channel openings was obtained from a sum of up to four Gaussian distributions fitted to these histograms by a least-squares method. The area under the Gaussians that corresponded to 1, 2, or 3 simultaneous channel opening was expressed as a proportion of the total area under the entire curve. The veracity of the fitting procedure was additionally checked by comparing the total area from the all-points histogram (sum of the bins) and the area under the fitted Gaussians (from the integral); these values differed by no more than 2%.
To measure channel open times and fit exponential distributions, the data files were imported into WinEDR (written by John Dempster, University of Strathclyde) for analysis. Channel openings were detected via a threshold set at 50% of amplitude of the single-channel current. Careful selection of stretches of data ensured that there were very few multiple-channel openings included in the analysis. To facilitate parameter estimation, the data were then plotted as a histogram with logarithmically increasing bin widths with 16 bins per decade [26].
Unless otherwise noted, the values are given as mean ± SEM and n values refer to the number of patches.
Results
A small conductance channel is sensitive to alterations in CO2
In the course of studying the CO2 sensitivity of connexins [11], we observed a small conductance channel in excised outside-out patches drawn from HeLa cells that exhibited sensitivity to changes in PCO2—the frequency of channel openings rapidly increased as the level of PCO2 increased (Fig. 1a, b). As extracellular pH was kept constant while PCO2 was changed (see “Methods”), the change in channel gating was unlikely to be due to alterations of extracellular pH. Equally changes in pH on the intracellular face of the membrane are also unlikely under this recording configuration as the excised patch has a very small membrane surface area, and it is improbable that CO2 would be able to diffuse through the membrane patch at a sufficient rate to rapidly alter the pH of the patch recording solution and hence channel gating. The CO2-dependent opening of the channel is thus most likely due to the direct effects of CO2 on the channel.
Increasing PCO2 increases open probability of a small conductance channel. a Continuous record of the effects of different CO2 concentrations on channel gating in outside-out patches from HeLa cells. Note that the effect of changing PCO2 from the control level of 35 mmHg to the levels marked on the black bars on channel gating was rapid. Isolated patch was held at +10 mV. b Expanded traces from a demonstrating the gating of the channels in the patch. Dotted lines represent different levels of channel openings, and multiple openings are only seen at the higher levels of PCO2. c All-points histograms obtained from the data in b and fitted with sums of Gaussian distributions to estimate P o. d Plot of P o vs PCO2 (n = 5 for each point; bars are SEMs). Continuous line is drawn to the Hill equation, P o = 1/(1 + (45/PCO2)2)
Increasing PCO2 from its control value (35 mmHg) to 55 or 70 mmHg caused a progressively bigger increase in the frequency of channel openings such that multiple overlapping channel openings could readily be seen at these higher levels of PCO2 (Fig. 1a–c). A reduction of PCO2 to 20 mmHg resulted in a decrease in the frequency of channel openings (Fig. 1a–c).
To quantify these effects we estimated the open probability (P o) of the channel at different levels of PCO2 (see “Methods”). Our analysis showed that P o increased with PCO2 and could be fitted by the Hill equation assuming a Hill coefficient of 2 and a half-maximal activation of the channel at a PCO2 of 45 mmHg (Fig. 1d).
Channel open time distribution
We examined the distribution of channel open times at different levels of PCO2. Under control conditions (PCO2 35 mmHg), this distribution could be fitted by either one or the sum of two exponential distributions. This demonstrated a main open state with a mean open time of 2.1 ± 0.5 ms (n = 5). However in two of these cases, fitting a second distribution with a longer mean time constant gave a statistically significantly better fit (Fig. 2). We noticed that the prevalence of these longer time openings increased at higher levels of PCO2. In one case, it was possible to measure the mean open times at all four levels of PCO2 (Fig. 2; Table 1). We found that while the short and long mean open times did not vary significantly at different levels of PCO2, the amplitude of the distribution with the longer mean open time scaled with PCO2 (Table 1). This analysis suggests that increased levels of CO2 may increase P o by promoting entry into a second open state that has a longer mean open time (measured over all levels of PCO2, 7.0 ± 1.0 ms, n = 3).
Channel open time distributions recorded at different levels of PCO2. Analysis of raw data from Fig. 1. The solid line is the combined fit of two exponential distributions, each shown separately as grey dashed lines. At each level of PCO2, the fitting of two exponential distributions gave a statistically significantly better fit than a single exponential (P < 0.02, F test). The effects of 55 and 70 mmHg on channel open times are probably underestimated as these were taken from stretches of data early in the application of the elevated PCO2 to minimize the occurrence of multiple-channel openings
The current–voltage relationship of the single-channel currents
We examined how the single-channel currents altered with voltage (Fig. 3a). The single-channel current exhibited inward rectification (Fig. 3b). The conductance of the channel was 6.7 ± 0.5 pS (calculated from the linear portion of the I–V relation, n = 4, Fig. 3b) and the single-channel currents reversed at –70 mV (Fig. 3a, b). This reversal potential lay between the K+ and Cl– equilibrium potentials calculated to be –93 mV and –20 mV, respectively. Interestingly P o showed little variation at different potentials (Fig. 3c).
Current–voltage characteristics and permeability of the CO2-sensitive channel. a Single-channel gating in outside-out excised patches during a series of 10 mV steps from +20 mV to –70 mV (left to right) in control 3 and 30 mM K+ aCSF at a PCO2 of 70 mmHg. b Current–voltage plots of the single-channel currents in 3 mM K+ (n = 4) and 30 mM K+ (n = 4) aCSF. The reversal potential changed from –70 mV to –9 mV and the slope conductance of the channel was increased by elevating extracellular K+. c Plot of the open probability (P o) against membrane potential for three excised patches, measured in 30 mM K+ aCSF. P o exhibited no voltage dependence. Bars are SEMs
We tested the nature of the permeant ion by altering extracellular K+ concentration. A tenfold increase in K+ concentrations moved the reversal potential to –9 mV (Fig. 3a, b). This shift in reversal potential was similar to that predicted by the Nernst equation, suggesting that this channel has high selectivity for K+. Interestingly, in the presence of elevated extracellular K+, the single-channel conductance increased to 11.5 ± 0.5 pS (calculated from the linear portion of the I–V relation, n = 4, Fig. 3b). The dependence of the single-channel conductance on extracellular [K+] is characteristic of Kir channels.
A tenfold reduction of external chloride had no effect on the reversal potential (–70 mV, n = 3), the single-channel inward rectification or conductance (Fig. 4a). However, we found that this reduction of the concentration of extracellular Cl– often lowered the holding current and reduced basal noise levels (Fig. 4b). Thus, there appeared to be a persistent Cl– current carried by a channel closely located to the K+ channel, which can obscure the gating of the K+ channel.
Colocalization of Cl– and K+ channels in excised patches. a Current–voltage relationship of single-channel currents recorded an outside-out patch in the presence of 15 mM Cl– in the medium at a PCO2 of 70 mmHg. The holding potential was changed from +40 to –70 mV in 10-mV steps (left to right). The inset shows the summary graph comparing the I–V relations for control (n = 7, black squares) and lowered Cl– (open circles, n = 3). Bars are SEMs. b A continuous current record from the patch before and during the application of low Cl– aCSF. Portions of the record in control aCSF (i) and low Cl– aCSF (ii) are shown below. Note that the reduction of extracellular Cl– ions reduces both the holding current and basal noise. Outside-out patch held at +10 mV
Discussion
Channels of the Kir family exhibit varying degrees of inward rectification show a single-channel conductance that varies with extracellular K+ concentration and are not blocked by TEA [9]. As these are all features of the channel that we have described, the CO2-sensitive channel is most probably an exemplar of the Kir family some of which, for example Kir1.1, exhibit only relatively weak inward rectification [29].
The sensitivity of K+ channels to fluctuations in pH is widespread and has received much attention with respect to possible physiological functions in chemosensing [6, 16, 17, 30]. Several members of the Kir family are sensitive to pH and acidification causes these channels to close hence giving depolarization. Some of these channels, especially Kir4.1 and 5.1, are favored candidates to participate in chemosensing [13, 31, 32]. However, other Kir channels (notably Kir1.1) are also present in both the carotid body and in areas of the medulla oblongata that participate in chemosensing [25, 31]. Interestingly, the Kir channel that we have described opens with increases in PCO2. Increasing levels of PCO2 under physiological conditions would normally cause both extracellular and intracellular acidification. The net effect of a combined change in pH and PCO2 (the more usual physiological circumstance) on this variant of the Kir family would therefore depend upon its relative sensitivity to changes in pH versus changes in PCO2. Different members of the Kir family are distinguishable on the basis of their pH sensitivity, for example Kir1.1 is insensitive to extracellular pH and requires intracellular acidification for closure [33]. However, it remains unknown whether an increase in PCO2 at constant intracellular pH will enhance current through Kir1.1 [33]. A common consensus is that alterations in PCO2 levels are sensed through consequent changes in either extracellular or intracellular pH. This is partly because until now there have been few mechanisms proposed by which CO2 could be detected directly. However, we have recently shown that CO2 can interact directly with connexins [11, 12] causing them to open and release ATP. Our present results suggest that at least one member of the Kir family also exhibits direct sensitivity to CO2. Interestingly although Kir channels are tetrameric, the relationship of P o versus PCO2 can be fitted with a Hill coefficient of 2 possibly indicating that only two molecules of CO2 need to bind to the channel to enhance opening. Our finding that elevated levels of PCO2 increase the occurrence of a second open state with a longer mean open time could imply that binding of CO2 to the channel promotes entry into this second open state.
How the K+ channel reported here might fit into a physiological system would depend on where it is expressed. In non-excitable cells of secretory tissues, opening of K+ channels and consequent K+ efflux causes secretion in exocrine glands; thus most secretions have elevated K+. CO2-dependent secretion involving a K+ channel has been found in tissue slices of the parotid gland, though this appears to be due to the stimulation of a second messenger [28] such as soluble adenylate cyclase [3, 35]. In pancreatic acinar cells, a low conductance (17 pS at symmetrical K+ concentrations) inwardly rectifying TEA-insensitive channel has been described [23, 24].
We commonly found that a Cl– current was also present in the excised patches along with the K+ channel. There is a precedent for Kir channels, notably Kir1.1, being localized with the CFTR Cl– channel in the apical membrane of cells in the thick ascending limb of the loop of Henlé in the kidney [9]. In many secretory systems, Cl– and K+ channels colocalize to ensure that K+ efflux from the cell is accompanied by chloride extrusion into the extracellular space [27]. Secretory cells such as parotid acinar cells express several chloride ion channels, which control extrusion and re-uptake of chloride ions from the cell [15].
Kir channels in the central nervous system help to control the resting potential. In all cases, so far reported Kir channels close in response to acidification, thus giving pH-dependent depolarization. The surprising implication of our results is that increasing CO2 would cause the opening of this particular Kir channel and hence lead to hyperpolarization. This could therefore be a mechanism that contributes to inhibitory processes occurring during hypercapnia. Such processes have been described. For example, slowly adapting pulmonary stretch receptors (SARs) are inhibited during hypercapnia by the activation of a TEA-insensitive potassium channel [14]. This inhibition has been previously attributed to alterations in extracellular pH as acetazolamide, a carbonic anhydrase inhibitor, significantly altered the response of SARs to CO2 [14]. Interestingly Kir channels, including Kir1.1, have been described in the NTS, an area where the SARs terminate onto their second-order cells [32]. Alternatively, this channel may play a role in the hyperpolarization of GABAergic and glycinergic neurons, which would lead to disinhibition of neural networks. Disinhibition occurs frequently in the cardiorespiratory network during hypercapnia [10]; it leads to a loss of glycinergic inputs in the cardioinhibitory vagal neurons and inspiratory-related GABAergic inputs [10]. These GABAergic inputs may come from the raphé magnus [1]. Were this channel expressed on these neurons, an increase in CO2 would inhibit them and remove the GABAergic input into the pre-Bötzinger, leading to an increase in respiration.
References
Cao Y, Fujito Y, Matsuyama K, Aoki M (2006) Effects of electrical stimulation of the medullary raphé nuclei on respiratory movement in rats. J Comp Physiol 192:497–505
Catron TF, Powell FL, West JB (2006) A strategy for determining arterial blood gases on the summit of Mt. Everest. BioMed Central Physiol 6:1–6
Chen Y, Cann MJ, Litvin TN, Lourgenko V, Sinclair ML, Levin LR, Buck J (2000) Soluble adenylyl cyclase as an evoultionary conserved bicarbonate sensor. Science 289:625–627
Feldman JL, Mitchell GS, Nattie EE (2003) Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26:239–266
Filosa JA, Putnam RW (2003) Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 53:C145–C155
Guyenet PG (2008) The 2008 Carl Ludwig lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105:404–416
Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK (2005) Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol 90:247–257
Haldane JS, Priestley JG (1905) The regulation of lung ventillation. J Physiol 32:225–266
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366
Huang Z-G, Griffioen KJS, Wang X, Dergacheva O, Kamendi H, Gorini C, Bouairi E, Mendelowitz D (2006) Differential control of central cardiorespiratory interactions by hypercapnia and the effect of prenatal nicotine. J Neurosci 26:21–29
Huckstepp RTR, Eason R, Sachdev A, Dale N (2010) CO2-dependent opening of connexin 26 and related β connexins. J Physiol 588:3921–3931
Huckstepp RTR, Id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N (2010) Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588:3901–3920
Jiang C, Xu H, Cui N, Wu J (2001) An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Respir Physiol 129:141–157
Matsumoto S, Takahashi T, Tanimoto T, Saiki C, Takeda M (1999) Effects of potassium channel blockers on CO2-induced slowly adapting pulmonary stretch receptor inhibition. J Pharmacol Exp Therap 290:974–979
Melvin JE (1999) Chloride channels and salivary gland function. Crit Rev Oral Biol Med 10:199–209
Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG (2004) Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7:1360–1369
Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA (2007) TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27:14049–14058
Peers C, Buckler KJ (1995) Transduction of chemostimuli by the type 1 carotid body cell. J Membr Biol 144:1–9
Pineda J, Aghajanian GK (1997) Carbon dioxide regulates the tonic activity of locus coeruleus neurons by by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77:723–743
Putnam RW, Filosa JA, Ritucci NA (2004) Cellular mechanisms involved in CO2 and acid signalling in chemosensitive neurons. Am J Physiol Cell Physiol 287:C1493–C1526
Richerson GB (2004) Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5:449–461
Ritucci NA, Erlichman JS, Leiter JC, Putnam RW (2005) Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol—Regulat Intergr Compar Physiol 289:851–861
Schmid A, Feick P, Schulz I (1997) Inwardly rectifying, voltage-dependent and resting potassium currents in rat pancreatic acinar cells in primary culture. J Physiol 504:259–270
Schmid A, Schulz I (1995) Characterization of single potassium channels in mouse pancreatic acinar cells. J Physiol 484:661–676
Schultz J-H, Czachurski J, Volk T, Ehmke H, Seller H (2003) Central sympathetic chemosensitivity and Kir1 potassium channels in the cat. Brain Res 963:113–120
Sigworth FJ, Sine SM (1987) Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J 52:1047–1054
Sørensen JB, Nielsen MS, Gudme CN, Larsen EH, Nielsen R (2001) Maxi K+ channels co-localised with CFTR in the apical membrane of an exocrine gland acinus: possible involvement in secretion. Pflügers Arch— Euro. J Physiol 442:1–11
Takahata T, Hayashi M, Ishikawa T (2003) SK4/IK1-like channels mediate TEA-insensitive, Ca2+-activated K+ currents in bovine parotid acinar cells. Am J Physiol Cell Physiol 284:C127–C144
Wang W-H (2006) Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290:F14–F19
Wellner-Kienitz M-C, Shams H, Scheid P (1998) Contribution of Ca2+-activated K+ channels to central chemosensitivity in cultivated neurons of fetal rat medulla. J Neurophysiol 79:2885–2894
Wu J, Xu H, Shen W, Jiang C (2004) Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol 197:179–191
Yamamoto Y, Ishikawa R, Omoe K, Taniguchi K (2008) Expression of inwardly rectifying K+ channels in the carotid body of rat. Histol Histopathol 23:799–806
Zhu G, Liu C, Qu Z, Chanchevalap S, Xu H, Jiang C (2000) CO2 inhibits specific inward rectifier K+ channels by decreases in intra- and extracellular pH. J Cell Physiol 183:53–64
Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, Welsh MJ, Wemmie JA (2009) The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139:1012–1021
Zippin JH, Levin LR, Buck J (2001) CO2/HCO –3 -responsive soluable adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab 12:366–370
Acknowledgments
We thank the Medical Research Council and Biotechnology and Biological Sciences Research Council for support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Huckstepp, R.T.R., Dale, N. CO2-dependent opening of an inwardly rectifying K+ channel. Pflugers Arch - Eur J Physiol 461, 337–344 (2011). https://doi.org/10.1007/s00424-010-0916-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-010-0916-z