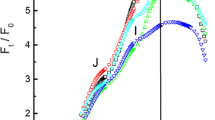
Abstract
Kinetic properties of the Na–Ca exchanger (guinea pig NCX1) expressed in Xenopus oocytes were investigated by patch clamp techniques and photolytic Ca2+ concentration jumps. Current measured in oocyte membranes expressing NCX1 is almost indistinguishable from current measured in patches derived from cardiac myocytes. In the Ca–Ca exchange mode, a transient inward current is observed, whereas in the Na–Ca exchange mode, current either rises to a plateau, or at higher Ca2+ concentration jumps, an initial transient is followed by a plateau. No comparable current was observed in membrane patches not expressing NCX1, indicating that photolytic Ca2+ concentrations jumps activate Na–Ca exchange current. Electrical currents generated by NCX1 expressed in Xenopus oocytes are about four times larger than those obtained from cardiac myocyte membranes enabling current recording with smaller concentration jumps and/or higher time resolution. The apparent affinity for Ca2+ of nonstationary exchange currents (0.1 mM) is much lower than that of stationary currents (6 μM). Measurement of the Ca2+ dependence of the rising phase provides direct evidence that the association rate constant for Ca2+ is about 5 × 108 M−1 s−1 and voltage independent. In both transport modes, the transient current decays with a voltage independent but Ca2+-dependent rate constant, which is about 9,000 s−1 at saturating Ca2+ concentrations. The voltage independence of this relaxation is maintained for Ca2+ concentrations far below saturation. In the Ca–Ca exchange mode, the amount of charge translocated after a concentration jump is independent of the magnitude of the jump but voltage dependent, increasing at negative voltages. The slope of the charge–voltage relation is independent of the Ca2+ concentration. Major conclusions are: (1) Photolytic Ca2+ concentration jumps generate current related to NCX1. (2) The dissociation constant for Ca2+ at the cytoplasmic transport binding site is about 0.1 mM. (3) The association rate constant of Ca2+ at the cytoplasmic transport sites is high (5 × 10−8 M−1s−1) and voltage independent. (4) The minimal five-state model (voltage independent binding reactions, one voltage independent conformational transition and one very fast voltage dependent conformational transition) used before to describe Ca2+ translocation at saturating Ca2+ concentrations is valid for Ca2+ concentrations far below saturation.










Similar content being viewed by others
References
Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Phys Rev 79:763–854
Diebler H, Eigen M, Ilgenfritz G, Maaß G, Winkler R (1969) Kinetics and mechanism of reactions of main group metal ions with biological carriers. Pure Appl Chem 20:93–115
DiPolo R, Beaugé L (1987) Characterization of the reverse Na/Ca exchange in squid axons and its modulation by Ca and ATP. Cai-dependent Nai/Cao and Nai/Nao exchange. J Gen Physiol 90:505–525
Ellis-Davies GCR, Kaplan JH, Barsotti RJ (1996) Laser photolysis of caged calcium: rates of calcium release by nitrophenyl-EGTA and DM-nitrophen. Biophys J 70:1006–1016
Haase A, Hartung K (2006) Activation and inactivation of Ca2+-activated Cl− current: photolytic Ca2+ concentration and voltage jump experiments. Pflügers Arch 452:81–90
Hilgemann DW (1989) Giant excised cardiac sacrolemmal membrane patches: sodium and sodium–calcium exchange currents. Pflügers Arch 415:247–249
Hilgemann DW (1990) Regulation and deregulation of cardiac Na+–Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature 344:242–245
Hilgemann DW (1996) Unitary cardiac Na+, Ca2+ exchange current magnitudes determined from channel-like noise and charge movements of ion transport. Biophys J 71:759–768
Hilgemann DW, Nicoll DA, Philipson KD (1991) Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature 352:715–718
Juhaszova M, Blaustein MP (1997) Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA 94:1800–1805
Kao JPY, Tsien RY (1988) Ca2+ binding kinetics of fura-2 and azo-1 from temperature-jump experiments. Biophys J 53:635–639
Kaplan J, Ellis-Davies GCR (1988) Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci USA 85:6571–6575
Kappl M, Hartung K (1996) Rapid charge translocation by the Na+–Ca2+ exchanger after a Ca2+ concentration jump. Biophys J 71:2473–2485
Kappl M, Nagel G, Hartung K (2001) Voltage and Ca2+ dependence of pre-steady-state currents of the Na–Ca exchanger generated by Ca2+ concentration jumps. Biophys J 81:2628–2638
Läuger P (1987) Voltage dependence of sodium–calcium exchange: predictions from kinetic models. J Membrane Biol 99:1–11
Matsuoka S, Hilgemann DW (1992) Steady-state and dynamic properties of cardiac sodium–calcium exchange: ion and voltage dependencies of the transport cycle. J Gen Physiol 100:963–1001
Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD (1995) Regulation of the cardiac Na–Ca exchanger by Ca2+. Mutational analysis of the Ca2+-binding domain. J Gen Physiol 105:405–420
Miledi R, Parker I (1984) Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol 357:173–183
Nicoll DA, Hryschko LV, Matsuoka S, Frank JS, Philipson KD (1996) Mutation of amino acid residues in the putative transmembrane segments of the cardiac sarcolemmal Na+–Ca2+ exchanger. J Biol Chem 271:13385–13391
Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD (1999) A new topological model of the cardiac sarcolemmal Na+–Ca2+ exchanger. J Biol Chem 274:910–917
Niggli E, Lederer W (1991) Molecular operations of the sodium–calcium exchanger revealed by conformation currents. Nature 349:621–624
Niggli E, Lipp P (1994) Voltage dependence of the Na-Ca exchanger conformational currents. Biophys J 67:1516-1524
Schlief T, Heinemann SH (1995) H2O2-induced chloride currents are indicative of an endogeneous Na+–Ca2+ exchanger mechanism in Xenopus oocytes. J Physiol 486:123–130
Schmies G, Engelhard M, Wood PG, Nagel G, Bamberg E (2001) Electrophysiological characterization of specific interactions between bacterial sensory rhodopsins and their transducers. Proc Natl Acad Sci USA 98:1555–1559
Wang S, George SE, Davis JP, Johnson JD (1988) Structural determinants of Ca2+ exchange and affinity in the C-terminal of cardiac troponin C. Biochemistry 37:14539–14544
Weinreich F, Wood PG, Riordan JR, Nagel G (1997) Direct action of genistein on CFTR. Eur J Physiol 434:484–491
Zucker RS (1993) The calcium concentration clamp: spikes and reversible pulses using the photolabile chelator DM-nitrophen. Cell Calcium 14:87–100
Acknowledgments
The authors thank Dr. C. Grewer for critical reading of the manuscript and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haase, A., Wood, P.G., Pintschovius, V. et al. Time resolved kinetics of the guinea pig Na–Ca exchanger (NCX1) expressed in Xenopus oocytes: voltage and Ca2+ dependence of pre-steady-state current investigated by photolytic Ca2+concentration jumps. Pflugers Arch - Eur J Physiol 454, 1031–1042 (2007). https://doi.org/10.1007/s00424-007-0260-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0260-0