Abstract
Background
Treatment of asymptomatic Abdominal Aortic Aneurysms (AAA) presents a clinical challenge, requiring a delicate balance between rupture risk, patient comorbidities, and intervention-related complications. International guidelines recommend intervention for specific AAA size thresholds, but these are based on historical trials with limited female representation. We aimed to analyse disease characteristics, AAA size at rupture, and intervention outcomes in patients with ruptured AAA from 2009 to 2023 to investigate the gap between guidelines and local realities.
Methods
This single-centre retrospective cohort study analysed electronic health records of patients treated for a ruptured AAA, excluding those who were managed palliatively. The study assessed patients’ demographics, risk factors, comorbidities, clinical presentation, radiological characteristics, and outcomes.
Results
Of 164 patients (41 females, 123 males, median age 73.5), 93.3% presented with abdominal or back pain. The median AAA size at rupture was 8.0 cm in males and 7.6 cm in females. No significant correlations were found between demographic characteristics, risk factors, AAA size, repair modality, and outcomes. Trends show a decline in AAA prevalence and rupture rates, aligning with global health initiatives. Post-intervention survival rates at 30 days were 70.7% (67.5% in males and 80.0% in females), and at 2 years were 65.85% (61.7% in males and 70.0% in females).
Conclusion
Evolving AAA trends and improved post-intervention survival rates warrant a critical reassessment of existing intervention recommendations. Adjusting intervention thresholds to larger sizes may be justified to optimise the risk-benefit ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of asymptomatic Abdominal Aortic Aneurysms (AAA) often poses a complex clinical dilemma, necessitating a nuanced balance between the risk of rupture, patients’ underlying comorbidities, and the potential intervention complications. The decision to intervene electively on AAA becomes more intricate in light of evidence asserting that long-term survival benefits, even after treatment, remain lower in patients with electively repaired AAA compared to their matched general populations without AAA [1,2,3]. The European Society for Vascular Surgery (ESVS) and the American Society for Vascular Surgery (SVS) recommend elective intervention when AAA reaches a threshold of 5.5 cm in men and 5.0 cm in women [4, 5]. Four randomised clinical trials were the cornerstone evidence for the development of these guidelines: two large multicentred randomised controlled trials of early open elective surgery versus surveillance, the UK Small Aneurysm Trial (UKSAT) and the American Aneurysm Detection And Management study (ADAM), and two smaller trials of endovascular repair versus surveillance; the Comparison of surveillance vs. Aortic Endografting for Small Aneurysm Repair (CAESAR) Trial, and the Positive Impact of endoVascular Options for Treating aneurysm early (PIVOTAL) study [6,7,8,9]. It is pertinent to acknowledge that the inclusion of a limited number of females in these trials has led to a reliance on comparatively weaker evidence for recommendations regarding female patients. The UKSAT reported an aneurysmal rupture rate of 1% per year in their cohort of patients with small aneurysms of 4.0–5.5 cm undergoing surveillance. It contrasts with a perioperative mortality of 5.6% in the immediate open repair cohort. Similarly, the ADAM study revealed a perioperative mortality of 2.7% in the open repair cohort compared to the rupture rate of 0.6% per year in the surveillance group for small AAAs (4.0 cm to 5.5 cm). These results affirm that immediate repair confers no advantage for small AAAs, irrespective of the chosen modality.
The current indication for intervention in AAAs larger than 5.5 cm is based on historical findings of alarming mortality rates associated with rupture —up to 80% in women and 70% in men [10]. ESVS 2011 guidelines indicated the yearly rupture risk escalating from 1% in AAA < 5.0 cm to 33% in aneurysms > 7.0 cm [11]. A recent meta-analysis scrutinising rupture rates based on size across 1514 patients from 11 studies indicates a significant departure from previous reports. Parkinson et al. found yearly rupture rates to be 3.5% in AAAs 5.5 to 6.0 cm, 4.1% in AAAs 6.1 to 7.0 cm, and 6.3% in AAAs > 7.0 cm [12]. Interestingly, the recently released ESVS 2024 guidelines acknowledge that adjusting this threshold to 6.0 cm may prove safe in the future as more evidence emerges. Their committee has therefore issued a strong negative recommendation for intervening electively on AAA < 5.5 cm and downgraded the recommendations for intervention at the 5.5 cm threshold to be a consideration of repair in men (from Class I to Class IIa) as the randomised clinical trials underlying this recommendation only showed that intervening on AAA < 5.5 cm is of no added benefit [13].
The lower rupture rates may be attributed to advancements in mitigating risk factors known to precipitate AAA rupture, including the decline in smoking prevalence and improved recognition and management of cardiovascular diseases [14, 15]. Furthermore, AAA screening programs implemented in various countries facilitate timely intervention and prevent AAA-related mortality. For instance, The 10-year follow-up results from the United Kingdom Multicentre Aneurysm Screening Study (MASS) revealed that screening men aged 65–74 for AAA led to a 48% relative risk reduction in deaths related to AAA over 10 years. The study demonstrated that the benefit of screening was maintained over time, with the mortality reduction seen in earlier years persisting in later years [16]. Similarly, in Denmark, a study focusing on asymptomatic men aged 64–73 years confirmed the favourable outcomes observed in the UK trials. The study demonstrated that screening for AAA resulted in a 66% reduction in AAA-related mortality at 14 years of follow-up [17, 18]. The systematic review and meta-analysis by Ying et al. consolidated evidence from randomised trials and cost-effectiveness analyses [19]. The review included five studies with a total of 175,085 participants aged 64 to 83 years, with a mean follow-up of 10.6 years. The results indicated that AAA screening led to a reduction in all-cause mortality, AAA-related mortality, and emergent AAA repair. The number needed to screen to prevent 1 AAA-related death per 10 years ranged from 209 to 769 individuals [19].
Considering these evolving trends in AAA prevalence, diagnosis and management, our retrospective single-centre cohort study aims to investigate the disease characteristics and AAA size at rupture for patients presenting to our regional vascular service at Auckland City Hospital from 2009 to 2023. The study further seeks to correlate these characteristics with patient outcomes, contributing valuable insights to the ongoing discourse on AAA management.
Methods
Study design
This retrospective cohort study investigates the demographic and disease characteristics of patients presenting with ruptured AAA between 2009 and 2023 at Auckland City Hospital. Patients were included from 2009 onwards to improve the accuracy of AAA measurements, as radiological modalities were better utilised during this period. Additionally, the study seeks to correlate these characteristics with patient outcomes. The study utilised electronic health records of patients diagnosed with ruptured AAA during this timeframe.
Study setting
The study was conducted at Auckland City Hospital, a single-centre tertiary care facility serving as one of at least 5 regional vascular surgery referral centres and equipped with advanced diagnostic and therapeutic facilities, including a hybrid theatre, for comprehensive management of vascular emergencies.
Data collection
Patients included in the study are 18 years or older, diagnosed with ruptured AAA between 2009 and 2023, and have complete records. Importantly, only patients who were deemed appropriate for surgical or endovascular intervention and subsequently treated were included. Patients managed palliatively without intervention were not included in this study. Patients were excluded from the study if they had definite evidence of mycotic rupture of AAA or non-ruptured AAA or if they presented with other aortic pathologies. Notably, two patients with rAAA and a diameter < 55 mm were included due to the lack of definitive evidence of mycotic aneurysm, despite the operating surgeon’s concern for mycotic features. Intra-operatively collected samples and repeated blood cultures during their inpatient stay showed no microbial growth, justifying their inclusion in this study. The following variables were collected: Demographics, including ethnicity, risk factors and comorbidities, clinical presentation, and radiological characteristics of AAA. AAA size at rupture as measured by ultrasound, CTA, or intra-operatively. Interventional data and peri-interventional complications. Mortality at 30 days, 90 days, and two years. Intensive care and inpatient hospital stay duration, and post-operative complications.
Limitations
Limitations of the study include the retrospective design, reliance on electronic health records, and the single-centre nature, which may impact the generalizability of findings. Furthermore, certain specific data points were not comprehensively collected due to limitations in the patient records, such as the intraoperative vasopressor support and the time from diagnosis to intervention. In addition, the size of AAA at rupture was obtained through variable modalities.
Statistical analysis
Descriptive statistics
These provided a comprehensive overview of the demographic and clinical characteristics of patients presenting with ruptured AAA from 2009 to 2023. This included measures of central tendency and dispersion for continuous variables and frequency distributions for categorical variables. Results were expressed as a median ± standard deviation (SD).
Correlation analysis
Statistical correlations were explored between patients’ sex and the size of AAA at rupture. Additionally, the relationship between sex and mortality outcomes was investigated. Similar correlation analyses were conducted with ethnicity as a variable to understand potential disparities in AAA size at rupture and outcomes among different ethnic groups.
Grouping analysis
Patients were grouped based on AAA size at rupture (< 5.5 cm, 5.6–6 cm, 6.1–7.0 cm, > 7.0 cm) and analysis to assess each group’s distribution and percentage representation. This grouping allowed for the exploration of the impact of AAA size on patient outcomes. In addition, maximum AAA diameter density plots were constructed to visually depict the distribution of AAA sizes at rupture within the cohort. Age density plots were generated to illustrate the age distribution of the patients. Furthermore, Kaplan-Meier estimates of survival were calculated to compare the survival rates of patients with ruptured AAA who survived at 30 days post AAA repair to those of a matched sex and year of birth in New Zealand. The New Zealand population for this comparative analysis was defined using national demographic data from Statistics New Zealand (Stats NZ), which includes age, sex, and ethnicity-specific mortality rates. This approach allowed for a meaningful comparison between the survival rates of our cohort and the general New Zealand population, accounting for key demographic factors [20]. This analysis enabled crude assessment of long-term survival trends following intervention.
Results
Descriptive statistics
In this study, 164 patients (41 females [25.0%] and 123 males [74.0%]) were enrolled, with a median age of 73.5 ± 16.53 (shown in Fig. 1). The ethnic distribution included 122 (74.4%) of European descent, 28 (17.1%) Māori, 11 (6.7%) Pacifica, and 3 (1.8%) of Asian ethnicity. Assessment of risk factors and comorbidities revealed that 53.0% had a history of regular smoking, 67.1% were hypertensive, 12.8% were diabetics, and 49.4% had a cardiac comorbidity. Unsurprisingly, most patients presented with abdominal or back pain (93.3%), 63.4% were hemodynamically unstable, defined as the presence of systolic blood pressure less than 90 mmHg, a heart rate exceeding 120 beats per minute, requirement for inotropic or vasopressor support, and clinical signs of shock such as altered mental status, oliguria, or cool extremities [21], and 5.5% suffered from end-organ dysfunction at presentation. The median size of AAA at rupture was 8.0 ± 1.87 cm in males and 7.6 ± 1.77 in females. The density plot for AAA maximum size at rupture is shown in shown in Fig. 2.
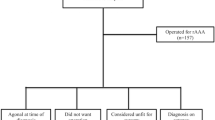
Patients were grouped based on the maximum diameter size at rupture: 3 patients (1.82%) had AAA < 5.5 cm, 17 patients (10.36%) had AAA sized 5.6–6.0 cm, 26 patients (15.85%) had AAA sized 6.1–7.0 cm, 103 (62.80%) had AAA of > 7.0 cm, and 15 (9.14%) had missing AAA size data on electronic records. CTA measured AAA sizes in 64.0% of patients, USS in 28.7%, and intra-operatively in 5.5% of patients. Notably, 18 patients (10.97%) experienced an interval rupture during surveillance, with 5 awaiting custom EVAR, 5 lost to follow-up, 3 declined elective intervention, 3 fell below the threshold for intervention, and 2 deemed unfit for intervention. The mean AAA diameters for patients experiencing interval rupture were 7.1 cm for males (n = 12) and 6.7 cm for females (n = 6).
Open surgical repair (OSR) was utilised in 136 (82.9%) patients, compared to 17 (10.4%) urgent Endovascular Aortic Repair (EVAR). The study found that the average use of EVAR per year for ruptured AAA (rEVAR) remained stable throughout the study duration, with an average of 1.2 rEVAR procedures performed per year. Eleven patients (6.7%) who proceeded for open repair arrested with failed resuscitation efforts “mors in tabula”.
Intra-operative details showed that proximal clamping was at the supra-coeliac level in 30.5% of patients, suprarenal in 12.8%, juxtarenal in 12.1%, and infrarenal in 44.7% of the patients. The median clamp duration was 22.5 ± 29.15 min. Patients who received OSR had proximal anastomosis at the supra-coeliac aorta (4.0%), juxtarenal (15.2%), or infrarenal aorta in 80.8% of the cases. The distal anastomosis sites were at the aortic bifurcation (56.6%), distal aorta (17.5%), common iliac artery (18.2%), internal iliac artery (0.7%), external iliac artery (4.2%), common femoral artery (12.3%), and the superficial femoral artery (0.7%). The median estimated blood loss was 3.1 ± 4.27 L, and the median operating duration was 170.0 ± 83.78 min.
Intra-operative complications occurred in 45 (27.4%) patients, manifesting as intra-operative cardiac arrest (8.5%), organ laceration (7.9%), torrential bleeding as described by the operator (7.3%), and intra-operative death (3.7%). In the study cohort, 44 (26.8%) returned to theatre; all patients who required a re-intervention were from the OSR group, with the exception of one patient in the EVAR group returning to theatre to control intra-abdominal bleeding. Return to theatre for abdominal closure was required in (6.7%) of patients, while (4.9%) returned to theatre to control bleeding. Furthermore, (7.9%) returned to theatre for bowel resection due to bowel ischemia. Post-operative complications were most pronounced in the OSR group compared to the EVAR group. These included cardiac events in (32.3%) of patients, while (44.5%) experienced a renal injury post-operatively, (36.6%) had a confirmed infective complication, (11.6%) developed mesenteric ischemia, and (4.9%) developed a stroke post-operatively. The percentage of these complications in the OSR and EVAR group, as well as the causes for return to theatre, is demonstrated in Fig. 3.
Overall, the 30-day survival rate was 70.7% (67.5% in males and 80.0% in females). In the OSR group, 75.0% and in the EVAR group, 88.2% survived 30 days post-operatively. No change in survival was observed at 90 days. The 2-year survival rate was 65.85% (61.7% in males and 70.0% in females), with 2.43% of patients lost to follow-up. Kaplan Meier survival estimates comparing patients who survived more than 30 days after intervention (n = 116) to the matched New Zealand population (shown in Fig. 4) showed a median survival post AAA rupture of 8.9 years compared to predicted NZ mean survival of 15.7 years (p < 0.001; Hazard ratio 4.38 +/- 0.23).
The median duration of intensive care stay was 3.0 ± 7.12 days, and the median inpatient duration was 11.5 ± 14.16 days. Discharge destinations included home (40.9%), transfer to another hospital (20.1%), a rehab facility (6.7%), and rest home discharge (1.8%). No significant trends in 30-day or 2-year survival rates, post-operative complications, duration of inpatient and intensive care stay, or discharge destinations were observed over the study period from 2009 to 2023.
Correlations among variables of interest
No statistically significant correlations were identified between patients’ sex or ethnicity and AAA size at rupture or mortality outcomes. Furthermore, no correlations were observed between risk factors such as smoking and hypertension and size at rupture or patient outcomes. In our patient population, neither the size of AAA at presentation nor the modality of repair yielded a statistically significant correlation with outcomes.
Discussion
Trends in AAA Prevalence and rupture Rates
The observed decline in AAA prevalence aligns with global efforts to improve health literacy and address key risk factors, particularly smoking [22]. Historical rates exceeding 5% are now substantially reduced to 1–2% and 1% in smoking females > 70 years old. This trend indicates the success of public health campaigns to mitigate risk factors associated with AAA [23,24,25]. As prevalence rates decline, a consequential decrease in rupture rates results in a significant shift from previous data. In the 1970s, rupture rates were reported to be 40% for AAAs > 6.0 cm, which decreased to 9.4% in the 1990s for AAAs 6.0 to 6.9 cm, and further reduced to 4.1% in AAAs 6.1 to 7.0 cm in recent studies [12, 26, 27].
Survival rates post rupture repair
Interventions for ruptured AAA have shown a gradual yet positive trend, with a noteworthy decrease in mortality rates observed over time. In our study, the 30-day survival rates were 75.0% in OSR and 88.2% in the EVAR group, while the overall 2-year survival rates were 65.9%. These outcomes align with recent studies reporting a 5-year survival of 68.8–71.0% in patients undergoing EVAR for ruptured AAA [28, 29]. In a recent review including data from IMPROVE and AJAX trials, 30-day survival rates were 64.6% for EVAR and 62.6% for OSR in IMPROVE. At one year, EVAR had a survival rate of 58.9%, while OSR was 54.9%. For AJAX, 30-day survival rates were 79% for EVAR and 75% for OSR. However, at six months, survival rates dropped to 72% for EVAR and 69.5% for OSR [30]. These results resonate with our study findings, indicating a modest yet notable improvement in survival outcomes compared to a previous literature review from 1991 to 2006, which reported 51% perioperative survival [31, 32]. The improved mortality rates and the recognised positive outcomes in high-volume centres indicate that ongoing regionalisation efforts in vascular surgery may lead to even better outcomes for these patients.
In this study, the higher ratio of OSR relative to rEVAR is in keeping with data from other centres in New Zealand. This trend can be attributed to several factors. Firstly, a significant proportion of our patients (63.4%) presented with hemodynamic instability, precluding many from undergoing the CTA necessary for EVAR planning. The urgent nature of their condition often necessitated immediate surgical intervention, favouring OSR over EVAR. Additionally, patient preference influenced the choice of OSR, although this aspect was not specifically documented in our study. During the earlier years of our study duration, the option for EVAR was limited due to infrastructure and the availability of trained personnel. While the introduction of emergency rEVAR in New Zealand presents an appealing alternative to open repair for rAAA, the adoption has been slow and gradual due to limited resources, patient load and endovascular experience [33]. The study found that the average use of EVAR per year for ruptured AAA remains stable, with an average of 1.2 rEVAR procedures performed per year. This stable trend indicates that despite the benefits of EVAR, it has not significantly increased over time in our setting. Globally, EVAR has shown increasing utilisation for rAAA. A review of nine countries reported an average annual rate increase of rEVAR from 5.3 to 15.1% between 2005 and 2009 [34], with current annual rEVAR rates of 35–70% within EVAR-capable centres in Sweden and the US [35, 36]. Local studies, such as the one by Peek et al. [37], highlighted that ruptured AAA repairs are more expensive than elective repairs, adding another layer of complexity to the decision-making process. The higher costs and logistical challenges associated with emergency EVAR might have contributed to the continued preference for OSR in acute settings. In summary, the combination of patient factors, historical limitations in infrastructure and expertise, and local economic considerations have collectively contributed to the higher ratio of OSR relative to rEVAR in New Zealand.
Considerations for intervention thresholds
The ongoing success in reducing AAA-related mortality prompts a critical re-evaluation of current international guidelines recommending intervention at a 5.5 cm or lower threshold. The latest ESVS guidelines recognise the changes in AAA trends and intervention outcomes, resulting in amending the wording from “recommending” elective intervention on AAA of 5.5 cm to “considering” intervention on AAA at this threshold [13]. Our data, reflecting a median size at rupture of 8 cm, suggests that the current guidelines may lead to an over-treatment of AAA. This is particularly relevant when considering the competing mortality risks of about 5% in elective repairs [38, 39]. Furthermore, this study highlights several factors that underscore the need for revisiting intervention thresholds. Firstly, despite international recommendations advising consideration of elective intervention at AAA thresholds of 5.5 cm, only 1.82% of our study population had a ruptured AAA at this size, with underlying aortic infection being a possible precipitating factor in 2 of the 3 patients. However, no definitive microbiological evidence could be elicited. Secondly, patient factors, including functional status and comorbidities, must be meticulously considered in determining the appropriateness of elective intervention. It is essential to recognise that patients with small AAAs may undergo a significant physiological insult during repair, potentially outweighing the benefits of intervening. Finally, our findings align with existing evidence indicating lower survival rates for patients with AAA, even after repair, compared to their matched populations. In our cohort, a notable proportion of patients presented with substantial comorbidities, including smoking diabetes, and importantly, 49% of our patient population had cardiac comorbidities. These comorbidities likely contributed to the observed poor long-term mortality rates, reflecting the overall health status and risk profiles of these patients rather than the outcomes of AAA repair alone. This study did not specifically match the patients’ comorbidities to the general population. However, the prevalence of cardiac comorbidities in patients over 60 years old is well-recognised [40]. Therefore, while comparing the long-term survival of patients with repaired rAAA to a demographics-matched population provides valuable insights, the potential bias introduced by the higher prevalence of comorbidities in the rAAA group must be considered and warrants future dedicated studies.
The study underscores the complexity of guiding AAA treatment solely based on aneurysm size and emphasises the importance of considering broader patient factors in decision-making. The observed trends in AAA prevalence, rupture rates, and post-intervention mortality outcomes suggest a potential need for revising current guidelines, prompting consideration for adjusting the elective intervention threshold to a larger size [41, 42]. It is important to note that our study is an observational and hypothesis-generating rather than a conclusive study. The findings should be interpreted within the context of a single-centre retrospective cohort study, and larger, multicentre studies are necessary to validate these observations and support any changes in clinical practice. Our discussion aims to highlight emerging trends and suggest a re-evaluation of current guidelines in light of these findings, not to recommend new thresholds definitively.
In conclusion, the dynamic nature of AAA disease trends and improved peri-intervention survival with lagging long-term survival benefits challenges existing paradigms. A careful reassessment of intervention thresholds is warranted.
Data availability
No datasets were generated or analysed during the current study.
References
Bahia SS, Holt PJ, Jackson D, Patterson BO, Hinchliffe RJ, Thompson MM, Karthikesalingam A (2015) Systematic review and Meta-analysis of long-term survival after Elective Infrarenal Abdominal aortic aneurysm repair 1969–2011: 5 year survival remains poor despite advances in Medical Care and Treatment strategies. Eur J Vasc Endovasc Surg 50(3):320–330 Epub 2015 Jun 23. PMID: 26116489; PMCID: PMC4831642
Bulder RMA, van der Vorst JR, van Schaik J, Bedene A, Lijfering WM, Bastiaannet E, Hamming JF, Lindeman JHN (2023) Persistent high long-term excess mortality after elective AAA repair especially in women: a large Population-based study. Ann Surg 278(5):815–822 Epub 2023 Jul 27. PMID: 37497631; PMCID: PMC10549885
Bulder RMA, Talvitie M, Bastiaannet E, Hamming JF, Hultgren R, Lindeman JHN (2020) Long-term Prognosis After Elective Abdominal Aortic Aneurysm Repair is Poor in Women and Men: The Challenges Remain. Ann Surg. ;272(5):773–778. https://doi.org/10.1097/SLA.0000000000004182. PMID: 32657926
Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z, Esvs G, de Committee GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F, Document Reviewers, Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein HH, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco JB, Verhagen H (eds) (2019) ‘s Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. ;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020. Epub 2018 Dec 5. Erratum in: Eur J Vasc Endovasc Surg. 2020;59(3):494. PMID: 30528142
Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW (2018) The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. ;67(1):2–77.e2. https://doi.org/10.1016/j.jvs.2017.10.044. PMID: 29268916
Powell JT, Greenhalgh RM, Ruckley CV, Fowkes FG (1996) The UK Small Aneurysm Trial. Ann N Y Acad Sci. ;800:249 – 51. https://doi.org/10.1111/j.1749-6632.1996.tb33320.x. PMID: 8959003
Brown LC, Thompson SG, Greenhalgh RM, Powell JT, UK Small Aneurysm Trial Participants (2008). Fit patients with small abdominal aortic aneurysms (AAAs) do not benefit from early intervention. J Vasc Surg. ;48(6):1375-81. https://doi.org/10.1016/j.jvs.2008.07.014. PMID: 19118733
Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E, CAESAR Trial Group (2011) Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg 41(1):13–25 Epub 2010 Sep 25. PMID: 20869890
Ouriel K (2009) The PIVOTAL study: a randomised comparison of endovascular repair versus surveillance in patients with smaller abdominal aortic aneurysms. J Vasc Surg. ;49(1):266-9. https://doi.org/10.1016/j.jvs.2008.11.048. PMID: 19174266
Schmitz-Rixen T, Böckler D, Vogl TJ, Grundmann RT (2020) Endovascular and open repair of abdominal aortic aneurysm. Dtsch Arztebl Int 117(48):813–819. https://doi.org/10.3238/arztebl.2020.0813PMID: 33568258; PMCID: PMC8005839
Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW, Rantner B, Schlösser FJ, Setacci F, Ricco JB, European Society for Vascular Surgery (2011). Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. ;41 Suppl 1:S1-S58. https://doi.org/10.1016/j.ejvs.2010.09.011. PMID: 21215940
Parkinson F, Ferguson S, Lewis P, Williams IM, Twine CP, South East Wales Vascular Network (2015) Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. J Vasc Surg 61(6):1606–1612. https://doi.org/10.1016/j.jvs.2014.10.023Epub 2015 Feb 7. PMID: 25661721
Wanhainen A, Van Herzeele I, Bastos Goncalves F, Bellmunt Montoya S, Berard X, Boyle JR, D’Oria M, Prendes CF, Karkos CD, Kazimierczak A, Koelemay MJW, Kölbel T, Mani K, Melissano G, Powell JT, Trimarchi S, Tsilimparis N(2024) ; ESVS Guidelines Committee; Antoniou GA, Björck M, Coscas R, Dias NV, Kolh P, Lepidi S, Mees BME, Resch TA, Ricco JB, Tulamo R, Twine CP; Document Reviewers; Branzan D, Cheng SWK, Dalman RL, Dick F, Golledge J, Haulon S van Herwaarden JA, Ilic NS, Jawien A, Mastracci TM, Oderich GS, Verzini F, Yeung KK. Editor’s Choice -- European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. ;67(2):192–331. https://doi.org/10.1016/j.ejvs.2023.11.002. Epub 2024 Jan 23. PMID: 38307694
Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, Lederle FA, Hunter DW, Bengtson LG, Guan W, Missov E, Folsom AR (2016) Lifetime risk and risk factors for abdominal aortic aneurysm in a 24-Year prospective study: the ARIC Study (Atherosclerosis Risk in communities). Arterioscler Thromb Vasc Biol 36(12):2468–2477 Epub 2016 Nov 10. PMID: 27834688; PMCID: PMC5397388
Gianfagna F, Veronesi G, Tozzi M, Tarallo A, Borchini R, Ferrario MM, Bertù L, Montonati A, Castelli P (2018) RoCAV (Risk of Cardiovascular diseases and abdominal aortic Aneurysm in Varese) Project Investigators. Prevalence of Abdominal Aortic Aneurysms in the General Population and in Subgroups at High Cardiovascular Risk in Italy. Results of the RoCAV Population Based Study. Eur J Vasc Endovasc Surg. ;55(5):633–639. doi: 10.1016/j.ejvs.2018.01.008. Epub 2018 Mar 3. PMID: 29506942
Thompson SG, Ashton HA, Gao L, Scott RA, Multicentre Aneurysm Screening Study Group (2009) Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 338:b2307. https://doi.org/10.1136/bmj.b2307PMID: 19553269; PMCID: PMC3272658
Lindholt JS, Juul S, Fasting H, Henneberg EW (2005) Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. ;330(7494):750. https://doi.org/10.1136/bmj.38369.620162.82. Epub 2005 Mar 9. Erratum in: BMJ. 2005;331(7521):876. PMID: 15757960; PMCID: PMC555873
Dabare D, Lo TT, McCormack DJ, Kung VW (2012) What is the role of screening in the management of abdominal aortic aneurysms? Interact Cardiovasc Thorac Surg 14(4):399–405. https://doi.org/10.1093/icvts/ivr106Epub 2012 Jan 19. PMID: 22268069; PMCID: PMC3309812
Ying AJ, Affan ET (2019) Abdominal Aortic Aneurysm Screening: A Systematic Review and Meta-analysis of Efficacy and Cost. Ann Vasc Surg. ;54:298–303.e3. https://doi.org/10.1016/j.avsg.2018.05.044. Epub 2018 Aug 4. PMID: 30081169
Statistics New Zealand (Year). Title of the webpage. Retrieved from https://nzdotstat.stats.govt.nz/wbos/Index.aspx
Coccolini F, Stahel PF, Montori G, Biffl W, Horer TM, Catena F, Kluger Y, Moore EE, Peitzman AB, Ivatury R, Coimbra R, Fraga GP, Pereira B, Rizoli S, Kirkpatrick A, Leppaniemi A, Manfredi R, Magnone S, Chiara O, Solaini L, Ceresoli M, Allievi N, Arvieux C, Velmahos G, Balogh Z, Naidoo N, Weber D, Abu-Zidan F, Sartelli M, Ansaloni L (2017) Pelvic trauma: WSES classification and guidelines. World J Emerg Surg 12:5. https://doi.org/10.1186/s13017-017-0117-6PMID: 28115984; PMCID: PMC5241998
Li X, Zhao G, Zhang J, Duan Z, Xin S (2013) Prevalence and trends of the abdominal aortic aneurysms epidemic in general population–a meta-analysis. PLoS ONE 8(12):e81260. https://doi.org/10.1371/journal.pone.0081260PMID: 24312543; PMCID: PMC3846841
Golledge J (2019) Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. ;16(4):225–242. https://doi.org/10.1038/s41569-018-0114-9. PMID: 30443031
Conway AM, Malkawi AH, Hinchliffe RJ, Holt PJ, Murray S, Thompson MM, Loftus IM (2012) First-year results of a national abdominal aortic aneurysm screening programme in a single centre. Br J Surg 99(1):73–77. https://doi.org/10.1002/bjs.7685Epub 2011 Sep 16. PMID: 21928466
Lilja F, Wanhainen A, Mani K (2017) Changes in abdominal aortic aneurysm epidemiology. J Cardiovasc Surg (Torino) 58(6):848–853. https://doi.org/10.23736/S0021-9509.17.10064-9Epub 2017 Jun 20. PMID: 28633519
Szilagyi DE, Elliott JP, Smith RF (1972) Clinical fate of the patient with asymptomatic abdominal aortic aneurysm and unfit for surgical treatment. Arch Surg. ;104(4):600-6. https://doi.org/10.1001/archsurg.1972.04180040214036. PMID: 5013802
Buth J (2000) Endovascular repair of abdominal aortic aneurysms. Results from the EUROSTAR registry. EUROpean collaborators on stent-graft techniques for abdominal aortic aneurysm repair. Seminars Interventional Cardiology: SIIC 5(1):29–33 PMID: 10875221
Oliveira-Pinto J, Soares-Ferreira R, Oliveira NFG, Bastos Gonçalves FM, Hoeks S, Van Rijn MJ, Raa ST, Mansilha A, Verhagen HJM (2020) Comparison of midterm results of endovascular aneurysm repair for ruptured and elective abdominal aortic aneurysms. J Vasc Surg. ;71(5):1554–1563.e1. doi: 10.1016/j.jvs.2019.07.091. Epub 2019 Oct 31. PMID: 31677942
Broos PP, ‘t Mannetje YW, Stokmans RA, Houterman S, Corte G, Cuypers PW, Teijink JA, van Sambeek MR (2016) A 15-Year single-center experience of Endovascular Repair for Elective and ruptured abdominal aortic aneurysms. J Endovasc Ther 23(4):566–573 Epub 2016 May 13. PMID: 27179252
Alnefaie SA, Alzahrani YA, Alzahrani BS (2022) A comparison of endovascular aneurysm repair and open repair for ruptured aortic abdominal aneurysms. Cureus 14(6):e25672. https://doi.org/10.7759/cureus.25672PMID: 35812617; PMCID: PMC9255951
Hoornweg LL, Storm-Versloot MN, Ubbink DT, Koelemay MJ, Legemate DA, Balm R (2008) Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 35(5):558–570 Epub 2008 Jan 15. PMID: 18226567
Bown MJ, Sutton AJ, Bell PR, Sayers RD (2002) A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. ;89(6):714 – 30. https://doi.org/10.1046/j.1365-2168.2002.02122.x. PMID: 12027981
Taylor S, Thomson I, Krysa J (2016) Emergency EVAR for ruptured abdominal aortic aneurysms: New Zealand experience. N Z Med J 129(1443):61–66 PMID: 27736853
2), Mani K, Lees T, Beiles B, Jensen LP, Venermo M, Simo G, Palombo D, Halbakken E, Troëng T, Wigger P, Björck M (2011) Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a vascunet report. Eur J Vasc Endovasc Surg 42(5):598–607 Epub 2011 Jul 19. PMID: 21775173
3), Mehta M, Byrne J, Darling RC 3rd, Paty PS, Roddy SP, Kreienberg PB, Taggert JB, Feustel P (2013) Endovascular repair of ruptured infrarenal abdominal aortic aneurysm is associated with lower 30-day mortality and better 5-year survival rates than open surgical repair. J Vasc Surg 57(2):368–375 Epub 2012 Dec 21. PMID: 23265582
4), Karthikesalingam A, Wanhainen A, Holt PJ, Vidal-Diez A, Brownrigg JR, Shpitser I, Björck M, Thompson MM, Mani K (2016) Comparison of long-term mortality after ruptured abdominal aortic aneurysm in England and Sweden. Br J Surg 103(3):199–206 Epub 2015 Dec 1. PMID: 26620854
5), Peek KN, Khashram M, Wells JE, Roake JA (2016) The costs of elective and emergency abdominal aortic aneurysm repair: a comparative single centre study. N Z Med J 129(1433):51–61 PMID: 27349161
Neal D, Beck AW, Eslami M, Schermerhorn ML, Cronenwett JL, Giles KA, Carroccio A, Jazaeri O, Huber TS, Upchurch GR Jr, Scali ST (2019) Validation of a pre-operative prediction model for mortality within 1 year after endovascular aortic aneurysm repair of intact aneurysms. J Vasc Surg 70(2):449–461e3. https://doi.org/10.1016/j.jvs.2018.10.122Epub 2019 Mar 25. PMID: 30922759
Filipovic M, Goldacre MJ, Gill L (2007) Elective surgery for aortic abdominal aneurysm: comparison of English outcomes with those elsewhere. J Epidemiol Community Health 61(3):226–231. https://doi.org/10.1136/jech.2006.047001PMID: 17325400; PMCID: PMC2652916
Zhou M, Zhao G, Zeng Y, Zhu J, Cheng F, Liang W (2022) Aging and Cardiovascular Disease: current Status and challenges. Rev Cardiovasc Med 23(4):135. https://doi.org/10.31083/j.rcm2304135PMID: 39076212; PMCID: PMC11274005
Resch TA, Eiberg J (2022) Are we over treating Abdominal aortic aneurysms? Eur J Vasc Endovasc Surg 64(6):592–594. https://doi.org/10.1016/j.ejvs.2022.10.009Epub 2022 Oct 8. PMID: 36216233
Thresholds for abdominal aortic aneurysm repair (2020 Mar) Abdominal aortic aneurysm: diagnosis and management: evidence review F. National Institute for Health and Care Excellence (NICE), London, p 32407032
Acknowledgements
Non applicable.
Funding
This study was not supported by any sponsor or funder.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
SH: Conceptualization, Data Collection, Writing - Original Draft. TF: Data Analysis, Writing - Review & Editing. RB: Supervision, Writing - Review & Editing.
Corresponding author
Ethics declarations
Statement of ethics
This study qualifies as an audit-related activity and was approved by the Vascular Surgery Department at Auckland City Hospital, with approval reference number. Patient confidentiality and data security were strictly maintained throughout the study. Written informed consent from participants was not required as this study was granted an exemption by the Vascular Surgery Department at Auckland City Hospital.
Conflict of interest
The authors have no conflicts of interest to declare.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, S., Frost, T. & Bourchier, R. Ruptured AAA: bridging the gap between international guidelines and local clinical realities. Langenbecks Arch Surg 409, 256 (2024). https://doi.org/10.1007/s00423-024-03441-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03441-6