Abstract
Purpose
To predict severe inflammatory response after neoadjuvant radiochemotherapy in locally advanced rectal cancer (RC) patients using magnetic resonance imaging (MRI) radiomics models.
Methods
This retrospective study included patients who underwent radical surgery for RC cancer after neoadjuvant radiochemotherapy between July 2017 and December 2019 at XXX Hospital. MRI radiomics features were extracted from T2WI images before (pre-nRCT-RF) and after (post-nRCT-RF) neoadjuvant radiochemotherapy, and the variation of radiomics features before and after neoadjuvant radiochemotherapy (delta-RF) were calculated. Eight, eight, and five most relevant features were identified for pre-nRCT-RF, post-nRCT-RF, and delta-RF, respectively.
Results
Eighty-six patients were included and randomized 3:1 to the training and test set (n = 65 and n = 21, respectively). The prediction model based on delta-RF had areas under the curve (AUCs) of 0.80 and 0.85 in the training and test set, respectively. A higher rate of difficult operations was observed in patients with severe inflammation (65.5% vs. 42.9%, P = 0.045).
Conclusion
The prediction model based on MRI delta-RF may be a useful tool for predicting severe inflammatory response after neoadjuvant radiochemotherapy in locally advanced RC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer (RC) ranks eighth among cancer incidences worldwide, with 732,210 new cases in 2020 and 339,022 deaths [1]. RC is considered to result from an accumulation of genetic and epigenetic alterations [2]. Many patients present with a locally advanced disease, which carries a 5-year survival rate of 71% [3]. The international standard treatment for locally advanced RC is total mesorectal excision after neoadjuvant radiochemotherapy [4,5,6,7,8]. Still, some tumors might be too large for resection or at risk of partial resection. Neoadjuvant therapy can shrink the tumor, thus improving the resection rate and patient prognosis [9,10,11]. Indeed, patients with locally advanced RC are difficult to treat with surgery alone, and a multidisciplinary approach is often necessary [8].
Radiochemotherapy can have side effects like inflammation and fibrosis [12, 13]. Chemotherapy induces tumor cell death that can lead to a local anti-tumor inflammatory effect [14,15,16]. The death of the tumor cells and the inflammatory microenvironment will also lead to the recruitment of myeloid cells that will initiate a kind of wound-healing process [15, 16]. The intensity of the inflammatory and fibrotic responses to radiochemotherapy appears to be associated with treatment efficacy, and a more severe response could indicate a more complete eradication of the tumor cells [15, 16]. At present, it is difficult for clinicians to observe by colonoscopy and traditional imaging whether a pathological complete response (pCR) or the inflammatory response of RC has been achieved after neoadjuvant therapy but before the operation. Nevertheless, evaluating such a response is important to help evaluate the effect of the neoadjuvant therapy. In addition, evaluating the inflammatory response could help determine whether the treatment is effective and formulate the management strategy.
Magnetic resonance imaging (MRI) has played an increasingly important role in diagnosing RC in recent years, often used as the gold standard for preoperative clinical staging of advanced RC. Usually, T2-weighted MRI sequences (T2WI) can clearly show hyper-intensity on the mucosal surface of the rectal and intestinal walls and hypo-intensity on the muscular layer of the intestinal wall. Still, the MRI-based determination of RC is affected by many factors, such as the skill level of the diagnostician, and it is often subjective [17,18,19]. As a non-invasive and effective means of prediction and recognition, radiomics-assisted diagnosis technology has great potential in cancer research [20, 21]. Recently, radiomics, especially MRI radiomics, has been extended to all aspects of the diagnosis and treatment of CRC, including distinguishing CRC from early cancer and predicting T stage, lymph node metastasis, peripheral nerve invasion, tumor treatment efficacy, microsatellite instability (MSI), and B-RAF status [22, 23]. The radiomics features (RFs) can be quantified to obtain a score, which can be compared pre/post-treatment to quantify the differences after treatment (delta-RF) [24, 25].
Still, presently, severe inflammatory response after neoadjuvant radiochemotherapy can only be confirmed by the histopathological examination of surgically removed specimens. Therefore, this study aimed to establish a prediction model based on MRI radiomics features to detect severe intestinal wall inflammatory response after neoadjuvant radiochemotherapy for locally advanced RC.
Methods
Study design and patients
This retrospective study included the patients who underwent radical surgery for RC cancer after neoadjuvant radiochemotherapy between July 2017 and December 2019 at XXX Hospital. This study was approved by the ethics committee of XXX Hospital. The requirement for individual informed consent was waived by the committee.
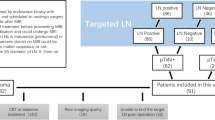
The inclusion criteria were 1) locally advanced rectal adenocarcinoma (preoperative cT3 or cT4 or cN +), 2) underwent standard radical resection of RC after neoadjuvant radiochemotherapy, and 3) underwent pelvic MRI examination before and at the end of neoadjuvant radiochemotherapy. The exclusion criteria were 1) multiple primary tumors, 2) interruption of radiochemotherapy or did not receive radiochemotherapy according to the treatment plan, or 3) incomplete data. The patients were randomized 3:1 to the training and test set (Fig. 1).
Treatment
The treatment plan and schedule of all patients followed the guidelines of the National Comprehensive Cancer Network (NCCN), which were current when the patients were treated. During the study period, the standard neoadjuvant radiochemotherapy regimen was intensity-modulated radiation therapy (IMRT) at 45–50 Gy in 25 fractions (1.8–2 Gy/fraction), which was completed in 35–42 days, combined with oral capecitabine 825 mg/m2, twice a day. Radical resection of rectal cancer was performed by colorectal surgeons with > 10 years of experience 6–9 weeks after neoadjuvant treatment. After surgery, all patients received oxaliplatin 130 mg/m2 d1 and capecitabine 1000 mg/m2 twice daily d1-14, every 21-day cycle, for 4–5 cycles (Fig. 2).
Data collection
This study collected the clinical and pathological indexes of the patients at baseline: age, sex, distance from the tumor to the anal margin, pathological differentiation, and TNM stage. All the data were from the medical records. The pathological characteristics and radiomics features were reappraised according to the surgical specimens and MRI imaging. The distance between the tumor and the anal margin was measured on the T2WI images as the distance between the lower edge of the tumor and the anal margin. The preoperative staging was revised according to the 8th edition of the AJCC Guidelines [26]. The patients whose operation time was < 205 min and whose intraoperative blood loss was < 60 ml were classified as routine operations, and the others were classified as difficult operations.
Pathological evaluation
Formalin-fixed paraffin-embedded tissue blocks were sectioned to 5 µm. The sections were stained using routine hematoxylin & eosin staining. The evaluation was performed by a pathologist with > 10 years of experience in diagnosing gastrointestinal tumors. The response of RC to neoadjuvant therapy was mostly shown as diffuse fibrosis with inflammatory cell infiltration. The pathological evaluation criteria referred to the 8th edition of the AJCC Guidelines [26]. The inflammatory response of the rectal wall where the tumor was located was graded according to the infiltration of inflammatory cells: grade 0 (absolutely no inflammatory response), grade 1 (mild inflammatory response), grade 2 (moderate inflammatory response), and grade 3 (severe inflammatory response). Grade 0–1 was considered mild inflammation, and grade 2–3 was severe (Fig. 3). The patients were divided into mild and severe inflammation according to the severity of the postoperative pathological inflammation.
Magnetic resonance imaging examination data
During the study period, the patients routinely underwent a pelvic enhanced MRI before radiochemotherapy, during treatment, and before radical surgery. All MRI examinations were performed using a 3.0-T Siemens magnetic resonance scanner, using an 8-channel phased array coil in the supine position. Conventional MRI sequences included sagittal T2WI, coronal T2WI parallel to the long axis of the lesion, pelvic T1WI, and diffusion-weighted imaging (DWI) with b values of 0 and 800 s/mm2, respectively. All MRI data were retrieved from the medical Picture Archiving and Communication System (PACS).
MR image acquisition parameters
All patients included in this study underwent high-resolution rectal MRI examination using a 3.0-T scanner. All examinations were performed using two devices: the GE MR Signa HDx 3.0 T and the Siemens MR Skyra 3.0 T. Patients fasted for more than 4 h before the MR examination. In addition to the routine contrast-enhanced rectal MR examination, all patients underwent a high-resolution, small field-of-view T2WI without fat suppression. For high-rectal and mid-rectal cancer, the imaging was performed perpendicular to the tumor segment. For low-rectal cancer, the imaging was done perpendicular to the tumor segment and parallel to the coronal plane of the anal canal. The parameters for the GE MR Signa HDx 3.0 T were as follows: 8-channel phased array torso coils; TR/TE = 5000/100 ms; FOV = 16 cm × 16 cm; NSA = 2; slice thickness = 3 mm; slice gap = 0.3 mm; matrix = 352 × 224. The parameters for the Siemens MR Skyra 3.0 T are as follows: 18-channel phased array torso coils; TR/TE = 5000/100 ms; FOV = 16 cm × 16 cm; NSA = 2; slice thickness = 3 mm; slice gap = 0.3 mm; matrix = 320 × 272.
Tumor delineation
The features were extracted from T2WI and DWI sequences. The imaging examinations were performed on T1WI sequences. The region of interest (ROI) was manually delineated in the MRI images by one radiologist with > 5 years of experience in the diagnosis of gastrointestinal tumors using ITK-SNAP (Fig. 2). The ROIs were selected based on clinical experience. Another imaging expert with > 10 years of experience diagnosing digestive tract tumors reviewed and revised the ROIs after discussion with the first radiologist. This study delineated a total of 432 levels of ROI, of which 3.5% were modified, suggesting high consistency. The selection of the ROI included all tumors and the corresponding rectal wall while avoiding blood vessels and intestinal gas. The T2WI images of RC show a slightly high signal before radiochemotherapy, and the ROI was placed in the area of high signal intensity. After radiochemotherapy, if low, mixed, or abnormal rectal wall signals were detected at the original tumor location on T2WI, the ROI was drawn with the abnormal signal area as the outline. If no abnormal or high signal was detected at the original tumor location on T2WI, the original tumor location area was used as the outline during ROI delineation.
Radiomics feature extraction
The Python 3.0 pyradiomics package was used to extract the RFs before (pre-nRCT-RF) and after (post-nRCT-RF) neoadjuvant radiochemotherapy, including first-order statistics feature (first order), GLCM (grey-level co-occurrence matrix), GLRLM (gray-level run-length matrix), GLSZM (gray-level size zone matrix), and GLDM (gray-level dependence matrix). The obtained radiomics features were standardized, with a total of 960 features. The variations of the radiomics features before and after neoadjuvant radiochemotherapy (delta-RF) were calculated using the following formulas: Delta-RF = (post-nRCT-RF—pre-nRCT-RF) / pre-nRCT-RF.
The redundant and irrelevant features were eliminated in advance using the minimum redundancy maximum relevance (mRMR) method. Using the mRMR algorithm, the top 20 features were selected from the original 960 features. Then, the least absolute shrinkage and selection operator (LASSO) was used to reduce the dimension and select the most valuable features as the input features of the final logistic regression model. The above work was completed using R 3.6.3.
The radscores were calculated for each patient by a linear combination of the selected features and coefficient vectors. The receiver operating characteristics (ROC) curves were drawn, and the areas under curves (AUCs) were calculated.
Statistical analysis
SPSS 22.0 (IBM, Armonk, NY, USA), Python 3.0, and R 3.6.3 were used for statistical analysis. All continuous data were tested for normal distribution using the Shapiro–Wilk test and conformed to the normal distribution; they were described as means ± standard deviations and analyzed using Student’s t-test. The categorical data were described as n (%) and analyzed using the chi-square test. The Wilcoxon signed-rank test was used to compare the differences in texture parameters before, during, and after neoadjuvant radiochemotherapy. Two-sided P < 0.05 were considered statistically significant.
Results
Demographic characteristic
Eighty-six patients were included in this study: 50 (58.1%) males and 36 (41.9%) females. Twenty-eight patients were included in the mild inflammation group, and 58 patients were included in the severe inflammation group; there were no significant differences in demographic and clinical characteristics between the two groups (all P > 0.05) (Supplementary Table S1). The patients were randomized 3:1 to the training and test set (n = 65 and n = 21, respectively). There were no significant differences in demographic and clinical characteristics between the mild and severe inflammation groups within each set (all P > 0.05) (Table 1).
The average operation time was 205 (135–320) min. The intraoperative blood loss was 60 (20–100) ml. A higher rate of difficult operations was observed in patients with severe inflammation (65.5% vs. 42.9%, P = 0.045) (Table 2).
Radiomics feature selection
A total of 960 radiomics features were obtained after standardized processing. The LASSO binary logistic regression model screened the eight, eight, and five most relevant features to predict the pathological inflammatory response for pre-nRCT-RF, post-nRCT-RF, and delta-RF (Fig. 4). The LASSO binary logistic regression model screened the five most relevant features (D_wavelet-LHH_glcm_MaximumProbabilty, D_wavelet-LLH_glcm_Correlation, D_Iog-sigma-3–0-mm-3D_firstorder_Median, D_oniginal_shape_Sphericity, D_log-sigma-3–0-mm-3D_firstorder_10Percentile) to predict the pathological inflammatory response for delta-RF.
Prediction model for the severe inflammatory response
The pre-nRCT-RF model predicted the pathological inflammatory response with AUC in the training and test set of 0.89 (95%CI: 0.814, 0.967) and 0.54 (95%CI: 0.286, 0.790), respectively (Fig. 5A-B). The pre-nRCT-RF model predicted the pathological inflammatory response with sensitivity and specificity in the training set of 0.786 and 0.87, respectively. The pre-nRCT-RF model predicted the pathological inflammatory response with sensitivity and specificity in the test set of 0.616 and 0.313, respectively.
Comparison of receiver operating characteristics (ROC) curves of the training and test set of three models. The radiomics before neoadjuvant radiochemotherapy (pre-nRCT) in the training (A) and test (B) set. The radiomics before radical surgery (post-nRCT) in the training (C) and test (D) set. The delta-radiomics in the training (E) and test (F) set
The post-nRCT-RF model predicted the pathological inflammatory response with AUCs in the training set and test set of 0.87 (95%CI: 0.771, 0.964) and 0.54 (95%CI: 0.229, 0.846), respectively (Fig. 5C-D). The post-nRCT-RF model predicted the pathological inflammatory response with sensitivity and specificity in the training set of 0.88 and 0.783, respectively. The post-nRCT-RF model predicted the pathological inflammatory response with sensitivity and specificity in the test set of 0.782 and 0.625, respectively.
The delta-RF model predicted the pathological inflammatory response with the AUCs in the training and test set of 0.80 (95%CI: 0.695, 0.910) and 0.85 (95%CI: 0.672, 1.000), respectively (Fig. 5E-F). The delta-RF model predicted the pathological inflammatory response with sensitivity and specificity in the training set of 0.667 and 0.826, respectively. The delta-RF model predicted the pathological inflammatory response with sensitivity and specificity in the test set of 0.813 and 0.8, respectively.
Discussion
There is a lack of radiomics research in predicting the side effects of radiochemotherapy for locally advanced RC. The present study performed a radiomics analysis on the pre-nRCT and post-nRCT MRI images of locally advanced RC. This study developed and evaluated the performance of a delta-RF model, which is based on the two MRI texture parameters to predict the inflammatory response of the intestinal wall after treatment, and the delta-RF model using T2WI best predicted inflammatory response. The results suggest that delta-RF is a potential imaging marker that can help clinicians guide their work and evaluate the side effects of neoadjuvant therapy for RC. Elaborating the most optimal treatment strategy involves gathering as much information about the tumor response as possible, including the inflammatory status of the lesion.
In this study, the pathological scores of inflammation in tissues around locally advanced RC after radiochemotherapy were used to classify the degree of tissue inflammation. The scoring system in this study is referred to as the tumor regression grading system. This system helps evaluate the treatment efficacy of neoadjuvant therapy [27]. According to the CSCO 2022 guidelines, TRG from 0 to 3 corresponds to different tumor regression states [28]. In the present study, the severity of inflammation was not associated with any demographic or clinical variables except with the complexity of the surgery. Therefore, age and sex were unrelated to the inflammatory response, suggesting that the inflammatory response after treatment is independent of those factors. Radiochemotherapy has acute effects on the tissues that are too important to be influenced by sex hormones or age. Although the efficacy and adverse effects of some chemotherapy regimens display differences between males and females, other regimens show similar adverse events; still, little is known about the sex disparities in adverse events to radiochemotherapy [29]. On the other hand, older age is often associated with frailty and lower functional reserves to face the adverse events of chemotherapy [30, 31]. Still, the sample size in the present study was relatively small, which could influence the results.
It has been suggested that the extent of the inflammatory and fibrotic responses of the tumor to radiochemotherapy appears to be associated with treatment efficacy [15, 16]. On the other hand, the inflammatory reaction can also affect the normal tissues around the tumor, which can increase the difficulty of the surgery [32]. Indeed, edema, exudation, and fibrosis can lead to unclear anatomical spaces, easy bleeding, and longer operation times for tissue dissociation, and edema can also result in longer anastomosis-related steps [33]. Therefore, a high tumor response to neoadjuvant radiochemotherapy can lead to a high inflammatory response of the tumor (associated with a good prognosis) and the surrounding tissues, complication surgery (associated with complications, morbidity, and a poorer prognosis). The present study showed a correlation between the severity of inflammation around the tumor and the difficulty of surgery, but there was no data regarding the oncological prognosis; additional studies will have to examine that issue. In addition, some studies suggested that the degree of inflammation in some patients will decrease when the waiting interval after neoadjuvant radiochemotherapy is prolonged [34,35,36,37,38]. Hence, it might suggest that the strategy and timing of surgery might be adjusted according to the inflammatory response. The timing of surgery for rectal cancer patients after radiochemotherapy requires finding a balance between oncology and inflammatory response. The present study was not designed to determine that timing and additional studies are necessary. Hence, even though the oncological impact might be limited, the impact on surgery could be non-negligible.
Delta-RF can capture rich information about the heterogeneous changes that may be discarded by single time point radiomics [39, 40]. Indeed, Wan et al. [39] used delta-radiomics to predict the pCR of RC to neoadjuvant radiochemotherapy. Jeon et al. [41] developed delta-radiomics models to predict local recurrence, distant metastasis, and disease-free survival in patients with RC. Other studies also showed the application of delta-RF in predicting the response to immunotherapy in patients with non-small-cell lung cancer [40] or neoadjuvant chemotherapy in breast cancer [42]. Although single-point radiomics can provide important information about a disease and even present predictive variables for treatment response, it cannot evaluate the response to the treatment. The advantage of delta-radiomics is precisely quantifying the imaging response to therapy. In the present study, delta-RF could predict the inflammatory response of the intestinal wall after treatment. Additional studies are necessary to examine the value of delta-RF and examine long-term outcomes like recurrence, metastasis, and survival.
This study has limitations. First, as a retrospective study, there were no external verifications of the results. It is necessary to conduct prospective studies with a larger sample size to confirm the findings. Second, tumor segmentation and texture feature extraction were only performed at a single layer. Although layer-by-layer segmentation can reflect the overall characteristics of tumors more comprehensively, its practical application in clinical practice is limited to a certain extent because it is time-consuming. Third, this study mainly focused on the texture parameters of T2WI, and it is necessary to study the predictive value of texture analysis using other sequences. Finally, it must be highlighted that radiomics examines hundreds of imaging features that are, most of the time, not even perceptible to the naked eye or with the help of traditional image examination software. Radiomics is often considered a “black box” that provides results based on the extraction and analysis software, but the parameters cannot be related to any apparent pathological or physiological feature [43,44,45].
In conclusion, a prediction model based on MRI delta-RF might predict severe inflammatory response after neoadjuvant radiochemotherapy in patients with advanced RC. The results might help surgeons better evaluate patients and optimize the operation.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to protecting individual patient privacy but are available from the corresponding author upon reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- cCR:
-
Clinical complete response
- CRC:
-
Colorectal cancer
- CSCO:
-
Chinese Society of Clinical Oncology
- delta-RF:
-
Variation of radiomics features before and after neoadjuvant radiochemotherapy
- DWI:
-
Diffusion-weighted imaging
- GLCM:
-
Gray-level co-occurrence matrix
- GLDM:
-
Gray-level dependence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLSZM:
-
Gray-level size zone matrix
- IMRT:
-
Intensity-modulated radiation therapy
- LASSO:
-
Least absolute shrinkage and selection operator
- MDT:
-
Multidisciplinary treatment
- MRI:
-
Magnetic resonance imaging
- MSI:
-
Microsatellite instability
- NCCN:
-
National Comprehensive Cancer Network
- PACS:
-
Picture archiving and communication system,
- pCR:
-
Pathological complete response
- PET:
-
Positron emission tomography
- pre-nRCT-RF:
-
Radiomics features before neoadjuvant radiochemotherapy
- post-nRCT-RF:
-
Radiomics features after neoadjuvant radiochemotherapy
- RC:
-
Rectal cancer
- RCT:
-
Radiochemotherapy
- RF:
-
Radiomics features
- ROC:
-
Receiver operating characteristics
- ROI:
-
Region of interest
- TME:
-
Total mesorectal excision
- TRG:
-
Tumor regression grade
- TR:
-
Repetition time
- TE:
-
Echo time
- FOV:
-
Field of view
- NSA:
-
Number of signal average
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG et al (2015) Colorectal Cancer Nat Rev Dis Primers 1:15065
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A (2023) Colorectal cancer statistics, 2023. CA Cancer J Clin 73(3):233–254
Montroni I, Ugolini G, Saur NM, Spinelli A, Rostoft S, Millan M et al (2018) Personalized management of elderly patients with rectal cancer: Expert recommendations of the European Society of Surgical Oncology, European Society of Coloproctology, International Society of Geriatric Oncology, and American College of Surgeons Commission on Cancer. Eur J Surg Oncol 44(11):1685–1702
Wo JY, Anker CJ, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT et al (2021) Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol 11(1):13–25
You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K et al (2020) The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum 63(9):1191–1222
De Bari B, Bosset JF, Gerard JP, Maingon P, Valentini V (2012) Multidisciplinary management of rectal cancer. Cancer Radiother 16(8):711–720
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) (2023) Rectal Cancer. Version 4.2022. Fort Washington: National Comprehensive CancerNetwork.http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
Martin LK, Bekaii-Saab T (2013) Optimizing neoadjuvant therapy for rectal cancer with oxaliplatin. J Natl Compr Canc Netw. 11(3):298–307
Reyngold M, Niland J, ter Veer A, Milne D, Bekaii-Saab T, Cohen SJ et al (2014) Neoadjuvant radiotherapy use in locally advanced rectal cancer at NCCN member institutions. J Natl Compr Canc Netw 12(2):235–243
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123
Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L et al (2004) Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 28(2):215–223
Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R (2007) Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol 188(2):442–451
Edwardson DW, Parissenti AM, Kovala AT (2019) Chemotherapy and Inflammatory Cytokine Signalling in Cancer Cells and the Tumour Microenvironment. Adv Exp Med Biol 1152:173–215
Hirata E, Sahai E (2017) Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb Perspect Med. 7(7):a026781
Merlano MC, Denaro N, Galizia D, Ruatta F, Occelli M, Minei S et al (2022) How Chemotherapy Affects the Tumor Immune Microenvironment: A Narrative Review. Biomedicines. 10(8):1822
Torkzad MR, Pahlman L, Glimelius B (2010) Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging 1(4):245–267
Saklani AP, Bae SU, Clayton A, Kim NK (2014) Magnetic resonance imaging in rectal cancer: a surgeon’s perspective. World J Gastroenterol 20(8):2030–2041
Kalisz KR, Enzerra MD, Paspulati RM (2019) MRI Evaluation of the Response of Rectal Cancer to Neoadjuvant Chemoradiation Therapy. Radiographics 39(2):538–556
Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z et al (2016) Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology 281(3):947–957
Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB et al (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30(9):1234–1248
Li W, Jiang Z, Guan Y, Chen Y, Huang X, Liu S et al (2018) Whole-lesion Apparent Diffusion Coefficient First- and Second-Order Texture Features for the Characterization of Rectal Cancer Pathological Factors. J Comput Assist Tomogr 42(4):642–647
Yang L, Dong D, Fang M, Zhu Y, Zang Y, Liu Z et al (2018) Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur Radiol 28(5):2058–2067
Cousin F, Louis T, Dheur S, Aboubakar F, Ghaye B, Occhipinti M et al (2023) Radiomics and Delta-Radiomics Signatures to Predict Response and Survival in Patients with Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Cancers (Basel). 15(7):1968
Abbas E, Fanni SC, Bandini C, Francischello R, Febi M, Aghakhanyan G et al (2023) Delta-radiomics in cancer immunotherapy response prediction: A systematic review. Eur J Radiol Open 11:100511
Weiser MR (2018) AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol 25(6):1454–1455
Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M et al (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 62(3):752–760
Diagnosis Treatment Guidelines For Colorectal Cancer Working Group C (2019) Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res 31(1):117–34
Rakshith HT, Lohita S, Rebello AP, Goudanavar PS, Raghavendra NN (2023) Sex differences in drug effects and/or toxicity in oncology. Curr Res Pharmacol Drug Discov 4:100152
Kim J, Hurria A (2013) Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 11(12):1494–1502
O’Donovan A, Leech M (2020) Personalised treatment for older adults with cancer: The role of frailty assessment. Tech Innov Patient Support Radiat Oncol 16:30–38
Nacion AJD, Park YY, Yang SY, Kim NK (2018) Critical and Challenging Issues in the Surgical Management of Low-Lying Rectal Cancer. Yonsei Med J 59(6):703–716
Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L et al (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16(2):200–207
Wasserberg N (2014) Interval to surgery after neoadjuvant treatment for colorectal cancer. World J Gastroenterol 20(15):4256–4262
Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M et al (2019) Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol 25(33):4850–4869
Yang J, Chen Q, Li J, Song Z, Cheng Y (2020) Short-Term Clinical and Oncological Outcome of Prolonging Operation Interval After Neoadjuvant Chemoradiotherapy for Locally Advanced Middle and Low Rectal Cancer. Cancer Manag Res 12:2315–2325
Mei SW, Liu Z, Wei FZ, Chen JN, Wang ZJ, Shen HY et al (2020) Impact of interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients. World J Gastroenterol 26(31):4624–4638
Yang Y, Huang A, Sun Z, Hong H-p, Kim NK, Gu J (2023) “Watch and wait” strategy after neoadjuvant chemoradiotherapy in rectal cancer: opportunities and challenges. Holistic Integrative Oncol 2(1):4
Wan L, Peng W, Zou S, Ye F, Geng Y, Ouyang H et al (2021) MRI-based delta-radiomics are predictive of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Acad Radiol 28(Suppl 1):S95–S104
Liu Y, Wu M, Zhang Y, Luo Y, He S, Wang Y et al (2021) Imaging Biomarkers to Predict and Evaluate the Effectiveness of Immunotherapy in Advanced Non-Small-Cell Lung Cancer. Front Oncol 11:657615
Jeon SH, Song C, Chie EK, Kim B, Kim YH, Chang W et al (2019) Delta-radiomics signature predicts treatment outcomes after preoperative chemoradiotherapy and surgery in rectal cancer. Radiat Oncol 14(1):43
Guo L, Du S, Gao S, Zhao R, Huang G, Jin F et al (2022) Delta-Radiomics Based on Dynamic Contrast-Enhanced MRI Predicts Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Cancers (Basel). 14(14):3515
Cobo M, Menendez Fernandez-Miranda P, Bastarrika G, Lloret IL (2023) Enhancing radiomics and Deep Learning systems through the standardization of medical imaging workflows. Sci Data 10(1):732
Zhang W, Guo Y, Jin Q (2023) Radiomics and Its Feature Selection: A Review. Symmetry 15(10):1834
Ibrahim A, Primakov S, Beuque M, Woodruff HC, Halilaj I, Wu G et al (2021) Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 188:20–29
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript.
Conceptualization: LC, WCZ.
Data curation: LC, EGC.
Formal analysis: LC, WZ, EGC.
Methodology: LC, EGC.
Resources: LC, EGC, WZ,
Software: LC, WCZ.
Writing – original draft: LC, WCZ.
Writing – review & editing: LC, WCZ, EGC.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the ethics committee of Sir Run Run Shaw Hospital Zhejiang University College of Medicine. This article is a retrospective study. Therefore, the institution waived the requirement to obtain distinct written informed consent from the patients.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L., Zhu, W., Zhang, W. et al. Magnetic resonance imaging radiomics-based prediction of severe inflammatory response in locally advanced rectal cancer patients after neoadjuvant radiochemotherapy. Langenbecks Arch Surg 409, 218 (2024). https://doi.org/10.1007/s00423-024-03416-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03416-7