Abstract
Purpose
The aim of this study was to report the outcomes of conversion surgery for initially unresectable advanced colorectal cancer and to identify factors that enable successful conversion surgery.
Methods
We compared the outcomes of patients with colorectal cancer with distant metastases, including extrahepatic metastases, who underwent upfront surgery, neoadjuvant chemotherapy, conversion surgery, and chemotherapy only at our department from 2007 to 2020. In addition, factors influencing the achievement of conversion surgery in patients who were initially unresectable were examined in univariate and multivariate analyses.
Results
Of 342 colorectal cancer patients with distant metastases treated during the study period, 239 were judged to be initially unresectable, and 17 (conversion rate: 7.1%) underwent conversion surgery. The prognosis for the conversion surgery group was better than that of the chemotherapy only group but worse than that of the upfront surgery group. In the conversion surgery group, the recurrence-free survival after resection was significantly shorter than that upfront surgery group and neoadjuvant chemotherapy group, and no patients have been cured. Among patients who were initially unresectable, left-sided primary cancer and normal CA19-9 level were identified as independent factors contributing to the achievement of conversion surgery in a multivariate analysis.
Conclusions
Although relapse after conversion surgery is common, and no patients have been cured thus far, overall survival was better in comparison to patients who received chemotherapy only. Among unresectable cases, patients with left-sided primary cancer and normal CA19-9 levels are likely to be candidates for conversion surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In cases of colorectal cancer, favorable outcomes can be anticipated through surgical intervention, even in the presence of distant metastases. The five-year survival rates after resection of liver, lung, and peritoneal metastases range from 45 to 60% [1], 30–68% [2], and 27–32% [3, 4], respectively. For unresectable cases, systemic chemotherapy is used, and with the remarkable advancements in chemotherapy, the median survival time is now almost three years [5]. Successful chemotherapy can render previously unresectable tumors resectable. Conversion surgery refers to surgical intervention that renders initially unresectable tumors resectable following treatment [6]. Given the potential for conversion surgery to improve prognoses [7], it is imperative to perform resection before the optimal window of opportunity elapses, even if the tumor is initially deemed unresectable. On the other hand, neoadjuvant chemotherapy is employed to improve the outcome after resection in resectable patients [8]. Because neoadjuvant chemotherapy and conversion surgery are similar in that surgery is performed after chemotherapy, they are often reported in a confusing manner. In this study, we report the results of upfront surgery, neoadjuvant chemotherapy, and conversion surgery for advanced colorectal cancer with distant metastasis, including extrahepatic metastasis, at our institution, and we aim to identify the factors that enable successful conversion surgery. We also raise questions regarding the definitions of conversion surgery and conversion rate.
Methods
Patients
Patients diagnosed with colorectal cancer who had distant metastasis and were treated at our department between 2007 and 2020 were included in this study. Resectability was determined based on two criteria: technical feasibility and preservation of vital organ function. The decision to proceed with resection was made after a group conference discussion. For cases deemed resectable, neoadjuvant chemotherapy was prescribed for patients with multiple organ or extensive distant lymph node metastases, as well as for cases with the possibility of exposing tumor tissue on the dissected surface (marginally resectable cases). The final decision was made by the attending physician, considering the patient’s overall health and preferences. Specific surgical procedures were determined by the hepatobiliary surgeon for liver metastases, by the respiratory surgeon for lung metastases, and the colorectal surgeon for the other cases. For peritoneal metastasis, the surgeon aimed for complete gross resection of the tumor, including resection of the dissemination nodule and concomitant resection of the surrounding organs if invasion was observed. Peritonectomy was not performed.
Patients were classified into four groups: those who underwent upfront surgery (Group S), those who received neoadjuvant chemotherapy before surgery (Group N), those who underwent conversion surgery (Group C), and those who received chemotherapy only because they were unresectable (Group I). The patients’ characteristics and prognoses were compared across these four groups.
Study items
Data regarding age, sex, primary site, metastatic organ, number of metastases, tumor markers, treatment received (e.g., surgery, neoadjuvant chemotherapy, conversion surgery, etc.), overall survival after the start of treatment, and postresection recurrence-free survival for patients who underwent R0 or R1 resection were extracted from medical records. Then, univariate and multivariate analyses were conducted to investigate the features of cases that were amenable to conversion surgery among those that were initially deemed unresectable. The surgical procedures, postoperative mortality, and morbidity were also assessed in Groups S, N, and C. Postoperative complications were evaluated using the Clavien-Dindo classification (CD), and the prognosis and the occurrence of CD grade ≥ 3 complications were compared.
Statistical analyses
We estimated survival curves using the Kaplan‒Meier method and compared them using a stratified log-rank test. For categorical variables, we used chi-squared and Fisher’s exact tests to compare clinical outcomes and proportions between groups as appropriate. For continuous variables, we used Wilcoxon tests. A logistic regression analysis was used for the multivariate analysis. P values of < 0.05 were considered to indicate statistical significance. Data were statistically analyzed using the JMP software program (version 16.2, SAS Institute Inc., Cary, NC, USA).
Results
During the review period, 342 cases of colorectal cancer with distant metastasis were treated at our department, with 64 cases in Group S, 39 cases in Group N, and 239 cases initially deemed unresectable. Of the unresectable cases, 17 were in Group C, achieving a conversion rate of 7.1%, while 222 cases were in Group I. The background of each group is provided in Table 1. In Group C, all but one had left-sided primary cancer. Five patients had metastasis to multiple organs and underwent extended surgery. There were significantly more cases in Group C with R1 than in Groups S and N (P < 0.0001).
The surgical procedures of Groups S, N, and C are listed in Table 2. With regard to the surgical procedure for liver metastases, extended hepatic resection with ≥ 4 segments was performed more frequently in Group C. The postoperative mortality and morbidity are shown in Table 3. There was 1 patient each in Groups S and C who died of complications, but there was no significant difference between the groups. The complication rates were also not significantly different between the groups.
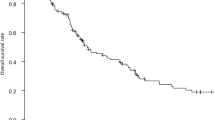
Figure 1 depicts the overall survival after the start of treatment, showing that Group S had a significantly better prognosis than Group C, while Group C had a significantly better prognosis than Group I. The prognosis of Group N was not significantly different from that of Group C. Figure 2 illustrates the relapse-free survival after resection in Groups S, N, and C where R0/R1 resection was performed, indicating that Group C had a significantly shorter RFS than Groups S and N. In Group C, 15 patients experienced recurrence; the exceptions were one patient who died of liver failure after surgery and one who died of other diseases. No patients achieved a cure. A comparison between the occurrence of postoperative complications and the prognosis for CD grade ≧ 3 is shown in Fig. 3. There was no significant difference overall or in Group S. The prognosis of patients with complications was significantly worse in Group N. In Group C, the prognosis tended to be worse in patients with complications; however, the difference was not statistically significant.
Survival curves compared by occurrence of postoperative complications. There was no significant difference overall (a) or in up-front surgery group (b). The prognosis of patients with complications was significantly worse in neoadjuvant chemotherapy group (c). In conversion surgery group (d), the prognosis tended to be worse in patients with complications; however, the difference was not statistically significant
When Group C was compared to Group I in patients initially deemed unresectable (Table 4), a univariate analysis showed that Group C had included significantly more male patients, patients with left-sided primary cancer, patients with single organ metastasis, patients with no peritoneal dissemination, and patients whose CA19-9 levels were within the normal range. In a multivariate analysis of these factors, a CA19-9 level within the normal range was independently associated with conversion surgery.
Discussion
Conversion rates in large clinical trials for unresectable advanced recurrent colorectal cancer have been found to be remarkably high. Several reports suggest that > 50% of colorectal cancers may be resectable, which may present a risk of disseminating misleading information to the public by implying that more than half of such colorectal cancers could be surgically treated even if initially deemed unresectable. The review by Bolhuis et al. [1]. focused exclusively on liver metastases in colorectal cancer and delineated the resectability criteria for such metastases. While the expected residual liver volume after resection is a criterion included in most reports, other criteria vary from trial to trial. In decision-making in relation to resectability, most reports involved a multidisciplinary team, but some reports indicated that the decision was made at a meeting of the medical team (as we made our decisions), while others did not describe the method of decision making. Given the inconsistencies in determining resectability and the criteria used, cases deemed unresectable in one trial may be judged as resectable in another [9]. We deem a tumor to be resectable if its resection is technically feasible and preservation of the vital organ function after resection can be ensured. Marginally resectable cases, in which the tumor on the dissected surface may be exposed but is not unresectable, are considered resectable. Preoperative chemotherapy for such cases is treated as neoadjuvant chemotherapy and when the tumor is resected it is not considered to be conversion surgery. Some reports treat cases that are technically resectable but oncologically unsuitable for resection as unresectable [10], so caution must be exercised when interpreting the conversion rate. If only cases that are technically unresectable and resected after chemotherapy are considered conversion surgeries, the actual conversion rate may be similar to the value we reported here.
Postoperative recurrence was frequently observed in patients who underwent conversion surgery in our study, and the RFS was significantly shorter than in patients who underwent upfront surgery or who underwent resection after neoadjuvant chemotherapy. Nonetheless, OS was better than in patients who received chemotherapy alone. Similar to the present investigation, Adam et al. reported that conversion surgery (termed “rescue surgery” in their report) exhibited a poorer prognosis in comparison to patients who received initial resection, but exhibited a more favorable outcome in comparison to unresectable cases [11]. If unresectable cases become resectable after chemotherapy, they may have a better prognosis in comparison to unresectable cases managed without surgery. However, surgery may be curative in such cases, and good outcomes that are difficult to achieve with chemotherapy alone have been reported [11, 12]. Based on a reanalysis of the cases in the Fire-3 trial, Modest et al. reported that the prognosis of patients who underwent surgery after being deemed resectable was better than that of patients who were deemed resectable but who did not undergo surgery [9]. Unfortunately, we have not yet experienced any curatively treated cases. However, considering the improvement in the prognosis, conversion surgery is still worthwhile, even though recurrence is frequently observed.
Conversion surgery is more likely to be invasive in patients with multiple metastases or invasion of adjacent organs due to the original unresectability of the disease. While there are reports on adverse events associated with chemotherapy administered before surgery, there are few reports on the safety of conversion surgery itself. Fukuoka et al. reported that liver resection after chemotherapy may be conducted safely without increased risk if the usual criteria are followed [13], thereby indicating that there is no requirement to exercise restraint in undertaking the procedure solely on the basis that it is performed after chemotherapy. Similarly, in the present study, chemotherapy had no effect on surgical safety. However, when the prognosis of patients with CD grade ≥ 3 complications was compared to the prognoses of the other groups, the prognosis of Group N was significantly worse, and the same trend was observed in Group C, although there was no significant difference in Group S (Fig. 3). The occurrence of postoperative complications has been reported to have a negative impact on the prognosis [14,15,16]; however, in this study, such an impact was only observed in post-chemotherapy cases. No reports have specifically addressed the prognostic impact of postoperative complications after chemotherapy, and future studies are warranted.
The multivariate analysis revealed that left-sided primary cancer and normal CA19-9 levels were the factors associated with the achievement of conversion surgery. Nozawa et al. reported single organ metastasis, liver metastasis cases, and use of molecular targeted therapy as independent factors [17], which differs from the results of our study. We extracted factors that are useful for prediction at the stage of treatment initiation without the addition of treatment factors, which may have contributed to the different results. Most other reports were limited to liver metastases, which makes it difficult to compare the results of the present study with those of other studies involving a wide range of unresectable colorectal cancer.
The present study was associated with several limitations, including the fact that it is a retrospective study of a small number of patients at a single institution. The small number of cases, especially in Group C, may have prevented a thorough statistical analysis. We believe that it is necessary to increase the number of cases and re-examine this issue in the future. While the liver is the most common site of metastasis in colorectal cancer, cases of liver metastasis alone are limited, and there are many cases of extrahepatic metastasis. The present study is significant because there have been few reports that included extrahepatic metastasis.
In conclusion, the present study reported that the conversion rate was 7.1% in patients with advanced colorectal cancer with distant metastasis, including unresectable nonhepatic metastasis, when technically unresectable was the criterion for unresectability. Notably, the prognosis was more favorable than that of unresectable cases treated solely with chemotherapy. Of note, among unresectable cases, patients with left-sided primary cancer and normal CA19-9 levels appear to be suitable candidates for conversion surgery.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Bolhuis K, Kos M, van Oijen MGH, Swijnenburg RJ, Punt CJA (2020) Conversion strategy with chemotherapy plus targeted agents for colorectal cancer liver-only metastases: a systemic review. Eur J Cancer 141:225–238
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T et al (2020) Japanese Society for Cancer of the Colon and rectum guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25(1):1–42
Kobayashi H, Kotake K, Funahiashi K, Hase K, Hirata K, Iiai T et al (2014) Clinical benefit of surgery for stage IV colorectal cancer with synchronous peritoneal metastasis. J Gastroenterol 49:646–654
Shida D, Tsukamoto S, Ochiai H, Kanemitsu Y (2018) Long-term outcomes after R0 resection of synchronous peritoneal metatasis from colorectal cancer without cytoreductive surgery or hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 25(1):173–178
Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H et al (2023) Panitumumab vs Bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type., left-sided metastatic colorectal cancer. JAMA 18(15):1271–1282
Maeda Y, Shinohara T, Nagatsu A, Futakawa N, Hamada T (2016) Long-term outcomes of conversion hepatectomy for initially unresectable colorectal liver metastases. Ann Surg Oncol 23:S242–S248
Adam R, Kitano Y (2019) Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol 3:50–56
Tanaka K, Adam R, Shimada H, Azoulay D, Levi F, Bismuth H (2003) Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg 90:963–969
Modest DP, Denecke T, Pratschke J, Ricard I, Lang H, Bemelmans M et al (2018) Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer-central evaluation of FIRE-3. Eur J Cancer 88:77–86
Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H et al (2014) Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol 21:S405–S413
Adam R, Delvart V, Pascal G, Valeanu A, Casraing D, Azoulay D et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy. Ann Surg 240(4):644–658
Delaunoit T, Alberts SR, Sargent DJ, Green E, Goldberg RM, Krook J et al (2005) Chemotherapy permits resection of metastatic colorectal cancer: experience from Intergroup N9741. Ann Oncol 16:425–429
Fukuoka K, Nara S, Honma Y, Kishi Y, Esaki M, Shimada K (2017) Hepatectomy for colorectal liver metastases in the era of modern preoperative chemotherapy: evaluation of postoperative complication. World J Surg 41:1073–1081
McArdle CS, McMillian DC, Hole DJ (2005) Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg 92:1150–1154
Khuri SF, Henderson WG, Depalma RG, Mosca C, Healey NA, Kumbhani DJ et al (2005) Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 242:326–343
Warps AK, Tollenaar RAEM, Tanis PJ, Dekker JWT (2022) Postoperative complications after colorectal cancer surgery and the association with long-term survival. Eur J Surg Oncol 48:873–882
Nozawa H, Ishihara S, Kawai K, Hata K, Kiyomatsu T, Tanaka T et al (2018) Conversion to resection in patients receiving systemic chemotherapy for unresectable and/or metastatic colorectal cancer-predictive factors and prognosis. Clin Colorectal cancer 17(1):e91–e97
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Gaku Ohira designed the study and wrote the initial draft of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Compliance with ethical standards
This study was approved by the institutional review board of Chiba University School of Medicine (No. 5–53). The opt-out recruitment method was applied to provide all patients an opportunity to decline to participate.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohira, G., Endo, S., Imanishi, S. et al. Prognosis and predictive factors of conversion surgery for initially unresectable advanced colorectal cancer. Langenbecks Arch Surg 409, 182 (2024). https://doi.org/10.1007/s00423-024-03374-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03374-0