Abstract
Introduction
Tracheoesophageal fistula (TEF) especially malignant TEF (mTEF) is an uncommon yet critical medical condition necessitating immediate intervention. This life-threatening condition frequently manifests in critically ill patients who are dependent on prolonged mechanical ventilation and are unsuitable candidates for thoracotomy due to their compromised health status. The Management of these mTEF patients remain a significant challenge.This study aimed to evaluate the safety and efficacy of using a cardiac septal occluder for the closure of mTEF.
Methods
8 patients with mTEF underwent closure surgery using atrial/ventricular septal defect (ASD/VSD) septal occluders at the Respiratory Department of HuBei Yichang Central People’s Hospital from 2021 to 2023. The procedure involved percutaneous placement of the occluder through the fistula to achieve closure.
Results
The placement of the cardiac septal occluder was successfully achieved with ease and efficiency in all patients. The study demonstrated that the use of cardiac septal occluder therapy in patients with mTEF can alleviate symptoms, improve quality of life, and enhance survival rates, with no significant complications observed. Furthermore, the study provided comprehensive details on surgical indications, preoperative evaluation and diagnosis, selection of occluder, methods of occlusion, and postoperative care.
Conclusions
The application of cardiac septal occluder in the treatment of mTEF is a safe and effective palliative treatment. This approach may be particularly beneficial for patients with a high risk of complications and mortality associated with traditional surgical interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant tracheoesophageal fistula (mTEF) is an aberrant pathological channel formed between the esophagus and trachea due to direct infiltration of malignant tumors or necrosis and perforation of the esophageal or tracheal walls during the course of primary tumor treatment [1]. mTEF commonly occurs as an advanced complication of malignant tumors, with a reported incidence rate of 5–15% in the past two decades [2]. Its main clinical manifestations include choking during swallowing, recurrent pulmonary infections, chronic cough with sputum production, respiratory distress, and weight loss [3]. Without appropriate intervention, mTEF can lead to mortality, with a median survival period ranging from 6 to 12 weeks [4]. The key to improving survival lies in the successful closure of the fistula, which is crucial for managing the two major life-threatening complications: pulmonary infection and malnutrition. Surgical treatment may not be suitable for patients with compromised health conditions, necessitating a personalized approach based on individual patient characteristics [4, 5].
Traditional surgical approaches for TEF closure, such as thoracotomy, have been associated with high mortality, particularly in elderly or frail patients due to their increased susceptibility to complications and prolonged hospital stays [6, 7]. However, minimally invasive techniques have emerged as alternative options for mTEF management, including endoscopic closure, stent placement, and laser-assisted repair etc. Nonetheless, the success rates of these minimally invasive techniques vary and depend on the size and location of the mTEF, as well as the overall health status of the patient [8]. The effectiveness and safety of these techniques are still being investigated, and the long-term outcomes are yet to be fully elucidated.
Currently, the application of cardiac septal occluder for the closure of TEF has been the subject of investigation in several studies, which have demonstrated promising outcomes in terms of safety and efficacy. This technique involves the percutaneous placement of a cardiac septal occluder to achieve closure of the fistula, thereby avoiding the need for extensive surgical dissection [9,10,11,12]. However, it should be noted that most of these studies lack standardized guidelines for the procedure, relying mainly on individual experiences. The feasibility of occlusion techniques for TEF has not been fully explored and requires further investigation [10]. Moreover, currently there are relatively few reported cases regarding the application of a cardiac septal occluder for the treatment of mTEF. In this study, we conducted a retrospective analysis of 8 cases involving patients with mTEF who underwent closure surgery using atrial/ventricular septal defect (ASD/VSD) septal occluder at the Respiratory Department of HuBei Yichang Central People’s Hospital from 2021 to 2023. Our findings demonstrated that the utilization of a cardiac septal occluder is a safe and effective approach for mTEF closure, with no significant complications observed. Additionally, we comprehensively summarized the closure surgical indications, preoperative evaluation and diagnosis, surgical instruments and materials, selection of occluder, methods of occlusion, and postoperative care. These findings provide valuable guidance for the clinical application of cardiac septal defect occluder in the management of TEF.
Materials and methods
Materials
The occlusion procedure involved the utilization of various materials and equipment, including a cardiac ASD/VSD septal occluder, a delivery sheath, a loading device, and a delivery cable (Chinese Medicine Saint Jie, Beijing, China), a 0.35” x 260 cm J-tip Emerald guidewire (Cordis 502455 Standard Tip), a rigid bronchoscope, a fiberoptic bronchoscope, an electrocardiography monitor, and a ventilator, among others [Fig. 1]. The selection of an appropriate occluder for TEF closure lacks standardized guidelines. The choice of the occluder size for each individual case should be carefully evaluated based on the location, size, and characteristics of the TEF.
Methods : procedure and technique
After obtaining approval from the Ethics Committee of Yichang Central People’s Hospital (Approval No. 2021-026-01), we conducted a retrospective analysis of data from eight patients with advanced mTEF who received treatment at the Respiratory Department of our hospital from 2021 to 2023. The patients included in the study underwent placement of a cardiac septal occluder under general anesthesia using a fiberoptic bronchoscope, with direct visualization for fistula occlusion. Written consent was obtained from all patients for both the research and publication purposes.
All the closure surgical procedures were performed under general anesthesia with deep sedation and analgesia, with continuous monitoring of the patient’s vital signs and blood oxygen saturation using electrocardiography. Initially, a rigid bronchoscope or laryngeal mask was inserted orally and connected to the ventilator for assisted respiration. Subsequently, the bronchoscope was used to confirm the location and size of the TEF opening on both the tracheal and esophageal sides. Under bronchoscopic guidance, a guidewire was passed through the bronchoscope forceps channel into the trachea, and then passed through the fistula into the esophagus[Figure 2A]. After removing the bronchoscope, the guide wire is used to guide the 45° ASD delivery sheath into the trachea, with the distal end passing through the fistula and into the esophagus to establish the surgical pathway (The size of the delivery sheath was chosen according to the actual size of the fistula) [Fig. 2B]. Next, the appropriate size cardiac septal occluder was preloaded onto a delivery cable and connected to it by rotating it clockwise using a screw connection system. The occluder was then sent into the esophagus through a delivery sheath [Fig. 2C]. The delivery sheath was then withdrawn, and the occluder was deployed under direct visualization through the esophagus. The occluder was gently pulled to fit snugly against the esophageal wall [Fig. 2D]. Subsequently, the bronchoscope was reintroduced into the trachea to slowly withdraw the delivery sheath under direct visualization and release the proximal occluder on the tracheal side [Fig. 2E]. The occluder was then gently pushed and pulled with the cable to ensure that it was fixed at the tracheoesophageal fistula, completely occluding the fistula and ensuring unobstructed passage of the trachea and esophagus. Finally, the cable was then rotated counterclockwise to detach it from the occluder, and both the cable and sheath were withdrawn to complete the surgery [Fig. 2F]. The entire surgical procedure takes approximately 30 to 60 min (Video 1).
Surgical procedure of treating mTEF with cardiac septal occlude. (A) Guide wire passes through bronchial fistula into esophagus; (B) Placement of delivery sheath; (C) Introducing cardiac septal occluder through fistula opening to esophagus via delivery sheath; (D) Positioning of distal end of occluder against the esophageal wall; (E) Counterclockwise rotation detaches cable from occluder; (F) Positioning of proximal end of occluder against the tracheal wall
Results
Clinical presentation
The study cohort comprised 8 patients with mTEF, with a median age of 72.9 years (range, 65–80 years) and a median body weight of 55.6 kg (range, 44–69 kg), including 6 males and 2 females. Among them, 5 patients (62.5%) were diagnosed with esophageal cancer, and 3 patients (37.5%) had lung cancer. All patients had a history of smoking and/or alcohol consumption. No other significant medical histories or surgical contraindications were noted. Hospital admission for these patients was primarily due to symptoms such as aspiration, coughing, inability to eat, and severe respiratory infections leading to sepsis.
All patients diagnosed with mTEF underwent a comprehensive preoperative diagnostic procedure, which included chest CT scan [Fig. 3A], bronchoscopy examination [Fig. 3B], and digestive endoscopy [Fig. 3C]. The mean size of the fistula opening was 8.6 mm (range, 6–14 mm). Surgical intervention for occlusion was indicated for mTEF cases with a diameter larger than 3 mm, assuming no significant tracheal lumen stenosis was present. Patients with mTEF who had the following contraindications were excluded from the study: severe cardiopulmonary insufficiency that would hinder the tolerability of minimally invasive bronchoscopy examination, uremia, recent myocardial infarction within one-month, cerebrovascular accidents, severe epilepsy, coronary heart disease, acute and chronic renal failure, severe hypertension, etc(Table 1). In our study, among the 8 patients who underwent successful closure of mTEF, 2 cases (25%) opted for ASD closure devices, while 6 cases (75%) chose VSD closure devices. The selection of the occluder size can be based on sizes commonly used for treating congenital heart disease, with the occluder size after expansion being greater than 3–4 mm.
Postoperative care and follow-up
Following mTEF occlusion, all patients should undergo close monitoring in the respiratory intensive care unit (RICU). Assisted ventilation may be required for 1 to 2days, and vigilant surveillance for signs of infection or bleeding is crucial. Antibiotics and other medications may be administered to prevent and treat infections, while analgesics can be given for effective pain management. Postoperative chest CT should be performed to ensure optimal placement of the occluder [Fig. 4]. Following the procedure, enteral feeding was initiated through a nasal feeding tube and continued for a duration of 15 to 30 days. The removal of the nasal feeding tube was considered after follow-up evaluation confirmed the secure fixation of the occlusion device with epithelialization and absence of leakage on esophagography. Once the nasal feeding tube was removed, the patient was able to resume oral intake. Patients may require extended hospital observation prior to discharge, and regular follow-up is essential to detect any potential complications, especially respiratory distress or airway obstruction. In the event of severe coughing, respiratory distress, or decreased blood oxygen saturation postoperatively, emergency bronchoscopy should be promptly performed to assess if the occlusion device has dislodged, potentially causing airway obstruction. If the detachment of the occluder is confirmed, immediate removal of the device should be performed under general anesthesia using methods such as forceps and mesh baskets via rigid bronchoscopy.
Long-term follow-up includes pulmonary CT, bronchoscopy, or digestive endoscopy to further assess the effectiveness of the occlusion, the status of the occluder endothelization, and the presence of pulmonary infections [Fig. 5]. the three-month postoperative follow-up, bronchoscopy examinations were conducted on all patients, revealing epithelialization of the occluder surface. Among the 8 patients included in the study, 6 patients (75%) exhibited complete endothelialization of the occluder after the closure procedure, while 2 patients (25%) showed partial endothelialization. No visible fistula openings were observed in either the esophageal or airway sides. Notably, there were no instances of coughing or aspiration, and a significant improvement was observed in pulmonary infections. Patients were able to eat without difficulties, and their overall quality of life showed remarkable improvement compared to the preoperative period. Importantly, all patients survived for a period exceeding 3 months (Table 2).
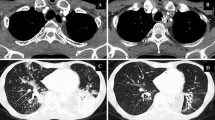
Follow-up evaluation 90 days after TEF occlusion in patients with esophageal cancer. (A) Gastroscopy examination showed epithelialization on the surface of the occluder, good adhesion to the esophageal wall, and no residual fistula; (B) After 90 days of TEF occlusion, chest CT was performed to confirm the position of the occluder. (C and D) Comparison of pre- and postoperative chest CT showed a significant improvement in pulmonary infection after TEF occlusion. The location of the occlusion device is indicated by the arrow
Discussion
TEF is a rare but life-threatening condition characterized by an abnormal communication between the trachea and esophagus, with an incidence of approximately 1 in 3,500 live births [13, 14]. In adults, TEF can occur as a result of trauma, malignancy, or iatrogenic injury. It is commonly observed in critically ill patients who require prolonged mechanical ventilation and are deemed unsuitable candidates for surgical intervention due to their compromised health status [4, 15]. The majority of TEF cases are located at the mid-tracheal level, predominantly on the posterior tracheal wall, and often present with respiratory infections caused by recurrent aspirations, sepsis, excessive secretions, coughing, and air leakage around the cuffed tube [16]. Spontaneous healing of TEF is rare, and surgical closure is typically required. Surgical repair has been the conventional treatment approach for TEF, involving the closure of the fistula through thoracotomy or laparotomy procedures [1, 17]. The optimal timing for surgery is after the patient has been successfully weaned off mechanical ventilation, the infection has been controlled, and optimal dietary conditions have been achieved [18, 19]. Despite its efficacy, surgical interventions are invasive and associated with a substantial risk of complications, including wound infection, pneumonia, and respiratory distress, especially for the mTEF [4, 20].
Various minimally invasive surgical approaches have been explored for the treatment of TEF. Endoscopic closure techniques involve the use of clips, sutures, or tissue adhesives to directly visualize and close the fistula under endoscopic guidance [21, 22]. Stent placement techniques entail the insertion of a self-expanding metal or silicone tube into the trachea or esophagus to maintain airway or esophageal patency [23,24,25,26]. Laser-assisted repair involves the controlled application of laser energy to create a burn and seal the fistula [27, 28]. Despite these minimally invasive techniques offer advantages such as reduced invasiveness and potentially faster recovery, their applicability and efficacy may vary depending on the individual case of TEF. The efficacy of bronchoscopic treatments using substances like fibrin glue, tissue adhesive, and sclerosing agents is limited when the size of the fistula exceeds 3 mm. Airway stents, including silicone stents and self-expandable metallic stents, have demonstrated some effectiveness [29]. However, due to the significant variation in tracheal diameter, stents may not adhere well to the tracheal mucosa, leading to incomplete fistula closure, stent dislocation, and the potential development of fatal tracheal stenosis [30,31,32]. The success rates of these approaches depend on factors such as the location, size, and nature of the fistula, as well as the overall health status and comorbidities of the patient. Consequently, careful consideration of the individual case is crucial when selecting the most appropriate treatment option especially in mTEF.
Recent studies have reported successful utilization of the cardiac septal occluder for TEF closure, particularly in patients who are not suitable candidates for traditional surgical approaches [12, 33, 34]. The cardiac septal occluder is a self-expanding, double-disc structure composed of tightly woven superelastic nickel-titanium alloy wire. It is primarily designed for closing cardiac septal defects such as atrial or ventricular septal defects. The occluder consists of two discs connected by a waist, with the diameters of the far and near discs being larger than the waist’s diameter. The edges of the discs are slightly concave, allowing them to interlock upon deployment, thereby enhancing the sealing effect. Additionally, a flow-blocking membrane is incorporated within the mesh structure of the occluder to reinforce the sealing capability[Figure 1C] [35].Its potential as a minimally invasive option for TEF treatment has garnered increasing attention. However, there is currently limited evidence regarding the application of cardiac septal occluders for the treatment of mTEF. Therefore, we conducted a retrospective analysis of 8 cases involving patients with mTEF who underwent closure surgery using atrial/ventricular septal defect (ASD/VSD) septal occluder at the Respiratory Department of HuBei Yichang Central People’s Hospital from 2021 to 2023.
In our study, stringent criteria were applied to select mTEF patients for occlusion surgery, taking into account their severe conditions such as advanced malignant tumors and overall poor health, which made conventional surgical intervention inappropriate. Additionally, comprehensive preoperative examinations played a pivotal role in patient selection. These adjunctive diagnostic procedures commonly employed in mTEF diagnosis not only facilitated prompt and accurate localization, sizing, and etiological identification of the fistula but also facilitated postoperative follow-up after occlusion therapy. Chest CT, bronchoscopy, and digestive endoscopy were the most frequently utilized adjunctive diagnostic procedures for TEF diagnosis. Successful execution of the occlusion surgery also necessitated collaborative efforts among respiratory physicians, cardiologists, gastroenterologists, anesthesiologists, and operating nurses.
Based on our experience in treating 8 cases of mTEF, we propose the use of an occluder waist diameter that is 3–4 mm larger than the length or diameter of the fistula to ensure complete coverage by the double discs. In terms of selecting ASD or VSD occluders for TEF closure, our study provides the following recommendations: for TEFs with a larger fistula diameter and larger esophageal and tracheal openings (> 1 cm), an ASD occluder is recommended. Conversely, for TEF with a smaller fistula diameter and smaller esophageal and tracheal openings (< 1 cm), a VSD occluder is preferred. VSD occluders are smaller and have a narrower waist compared to ASD occluders. The appropriate selection of occluders is crucial as an oversized occluder may compromise tissue circulation, leading to tissue damage and potential complications, while an undersized occluder may result in displacement or detachment. The complications associated with cardiac septal occluder treatment for malignant tracheoesophageal fistula encompass occluder displacement, tissue damage, infection, re-intervention, and persistent symptoms such as dysphagia and dyspnea. Occluder displacement poses a risk of inadequate closure, potentially leading to symptom recurrence, while insertion and deployment of the device may cause tissue damage, inflammation, or perforation. The presence of the occluder increases the risk of infection, necessitating antibiotic therapy. In cases of complications, re-intervention may be required to address issues such as occluder migration or inadequate closure, prolonging the recovery period and increasing the risk of further complications. Persistent symptoms such as difficulty swallowing or breathing may persist due to inadequate closure or occluder-related issues, impacting the patient’s quality of life and requiring additional management strategies.
Postoperative follow-up assessments revealed that the utilization of a cardiac septal occluder is a safe and effective approach for mTEF closure. Despite the three-month follow-up, two patients still exhibited partial endothelialization of the occluder. This is primarily attributed to the use of larger cardiac septal occluders due to the presence of a large mTEF. However, it is noteworthy that there was a significant improvement in pulmonary infections, allowing patients to eat without difficulty. Moreover, there was a remarkable enhancement in their overall quality of life compared to the preoperative period. Furthermore, no major complications were observed, and patients did not experience choking or aspiration following mTEF closure surgery. The utilization of the cardiac septal occluder offers several advantages, including shorter hospital stays and faster recovery times compared to traditional surgical techniques, with fewer postoperative complications such as wound infections and scarring. Importantly, the survival period exceeded the median survival period of 6 to 12 weeks.
Conclusions
In conclusion, that the use of a cardiac septal occluder could potentially emerge as a minimally invasive palliative alternative for treating mTEF. This approach shows promise as a treatment option for TEF closure, with high success rates and minimal complications. However, large-scale studies are needed to evaluate the long-term outcomes and safety of this technique [36]. Further clinical studies are warranted to establish standardized protocols, assess long-term outcomes, and compare the effectiveness and safety of the cardiac septal occluder with other minimally invasive techniques and traditional surgical approaches.
Data availability
No datasets were generated or analysed during the current study.
References
Walk RM (2022) Esophageal atresia and Tracheoesophageal Fistula: overview and considerations for the General Surgeon. Surg Clin North Am 102(5):759–778
Stamatis G, Freitag L (2011) [Tracheoesophageal fistula]. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 82(2):148–153
Lee S (2018) Basic Knowledge of Tracheoesophageal Fistula and Esophageal Atresia. Adv Neonatal care: Official J Natl Association Neonatal Nurses 18(1):14–21
Shamji FM, Inculet R (2018) Management of malignant Tracheoesophageal Fistula. Torac Surg Clin 28(3):393–402
Dingemann C, Eaton S, Aksnes G, Bagolan P, Cross KM, De Coppi P, Fruithof J, Gamba P, Husby S, Koivusalo A et al (2020) ERNICA Consensus Conference on the Management of Patients with Esophageal Atresia and Tracheoesophageal Fistula: Diagnostics, Preoperative, Operative, and Postoperative Management. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery = Zeitschrift fur Kinderchirurgie 30(4):326–336
Wang QX, Shi RH, Duan ZH (2022) [Management advances of malignant tracheoesophageal fistula]. Zhonghua Nei Ke Za Zhi 61(5):598–602
Hurtgen M, Herber SCA (2014) Treatment of malignant tracheoesophageal fistula. Torac Surg Clin 24(1):117–127
Kim HS, Khemasuwan D, Diaz-Mendoza J, Mehta AC (2020) Management of tracheo-oesophageal fistula in adults. Eur Respiratory Review: Official J Eur Respiratory Soc 29(158)
Rabenstein T, Boosfeld C, Henrich R, Ell C (2006) First use of ventricular septal defect occlusion device for endoscopic closure of an esophagorespiratory fistula using bronchoscopy and esophagoscopy. Chest 130(3):906–909
Jiang P, Liu J, Yu D, Jie B, Jiang S (2015) Closure of Nonmalignant Tracheoesophageal Fistula using an atrial septal defect occluder: Case Report and Review of the literature. Cardiovasc Interv Radiol 38(6):1635–1639
Bawaadam HS, Russell M, Gesthalter YB (2022) Acquired Benign Tracheoesophageal Fistula: Novel Use of a nasal septal occluder. J Bronchol Interventional Pulmonol 29(3):e38–e43
Silva JC, Braga P, Coutinho D, Fernades C (2020) Refractory tracheoesophageal fistula management with Amplatzer Occluder(R) placement. Rev Esp Enferm Dig 112(9):733–734
van der Zee DC, Tytgat SHA, van Herwaarden MYA (2017) Esophageal atresia and tracheo-esophageal fistula. Semin Pediatr Surg 26(2):67–71
Durkin N, De Coppi P (2022) Management of neonates with oesophageal atresia and tracheoesophageal fistula. Early Hum Dev 174:105681
McGowan NA, Grosel J (2022) An overview of esophageal atresia and tracheoesophageal fistula. JAAPA: Official J Am Acad Physician Assistants 35(6):34–37
Alslaim HS, Banooni AB, Shaltaf A, Novotny NM (2020) Tracheoesophageal fistula in the developing world: are we ready for thoracoscopic repair? Pediatr Surg Int 36(5):649–654
Osho A, Sachdeva U, Wright C, Muniappan A (2018) Surgical management of tracheoesophageal fistula. Annals Cardiothorac Surg 7(2):314–316
Salik I, Paul M (2023) Tracheoesophageal Fistula. In: StatPearls Treasure Island (FL) ineligible companies. Disclosure: Manju Paul declares no relevant financial relationships with ineligible companies
Lal DR, Gadepalli SK, Downard CD, Ostlie DJ, Minneci PC, Swedler RM, Chelius TH, Cassidy L, Rapp CT, Billmire D et al (2018) Challenging surgical dogma in the management of proximal esophageal atresia with distal tracheoesophageal fistula: outcomes from the Midwest Pediatric surgery Consortium. J Pediatr Surg 53(7):1267–1272
Sharma A, Rehman MU, Cowen ME (2003) Management of a difficult malignant tracheoesophageal fistula. Interact Cardiovasc Thorac Surg 2(4):665–667
Ramai D, Bivona A, Latson W, Ofosu A, Ofori E, Reddy M, Adler DG (2019) Endoscopic management of tracheoesophageal fistulas. Annals Gastroenterol 32(1):24–29
Richter GT, Ryckman F, Brown RL, Rutter MJ (2008) Endoscopic management of recurrent tracheoesophageal fistula. J Pediatr Surg 43(1):238–245
Wang QX, Duan ZH, Liu SQ, Feng YD, Shi RH (2023) [Efficacy of stent placement in treatment of malignant tracheoesophageal fistula and relevant factors of fistula closure]. Zhonghua Yi Xue Za Zhi 103(2):111–116
Mittal S, Madan K, Mohan A, Tiwari P (2021) Massive gastric distension following tracheobronchial Y-shaped self-expanding metallic stent placement for large tracheoesophageal fistula. Lung India: Official Organ Indian Chest Soc 38(1):92–93
de Vega Sanchez B, Vicente CD, Perez-Miranda M (2021) Triple stent placement for tracheoesophageal fistula closure. Arch Bronconeumol 57(11):698
Holmes MJV, George A (2022) Technical note: the use of a silicone prosthesis for the closure of a tracheoesophageal fistula. Clin Otolaryngology: Official J ENT-UK ; Official J Neth Soc Oto-Rhino-Laryngology Cervico-Facial Surg 47(3):483–485
Luscan R, Simon F, Khen Dunlop N, Gaudin R, Rousseau V, Couloigner V, Leboulanger N, Denoyelle F, Garabedian EN, Thierry B (2021) Thulium LASER for endoscopic closure of tracheoesophageal fistula in esophageal atresia’s spectrum: an appropriate tool? J Pediatr Surg 56(10):1752–1756
Bottero E, Manassero E, De Lorenzi D (2019) Diode laser treatment in a case of congenital tracheoesophageal fistula in a young dog. Can Veterinary J = La Revue Veterinaire Canadienne 60(5):472–476
Debourdeau A, Gonzalez JM, Dutau H, Benezech A, Barthet M (2019) Endoscopic treatment of nonmalignant tracheoesophageal and bronchoesophageal fistula: results and prognostic factors for its success. Surg Endosc 33(2):549–556
Verschuur EM, Kuipers EJ, Siersema PD (2007) Esophageal stents for malignant strictures close to the upper esophageal sphincter. Gastrointest Endosc 66(6):1082–1090
Herzog M, Greiner I (2011) Treatment of large pharyngotracheal fistulas after laryngectomy by a novel customized pharyngeal stent. Eur Archives oto-rhino-laryngology: Official J Eur Federation Oto-Rhino-Laryngological Soc 268(5):747–754
Tomaselli F, Maier A, Sankin O, Pinter H, Smolle J, Smolle-Juttner FM (2004) Ultraflex stent–benefits and risks in ultimate palliation of advanced, malignant stenosis in the esophagus. Hepatogastroenterology 51(58):1021–1026
Ersoz H, Nazli C (2018) A new method of tracheoesophageal fistula treatment: using an atrial septal defect occluder device for closure-the first Turkish experience. Gen Thorac Cardiovasc Surg 66(11):679–683
Siboni S, D’Aiello AF, Chessa M, Bonavina L (2022) Tailored endoscopic treatment of tracheo-oesophageal fistula using preoperative holographic assessment and a cardiac septal occluder. BMJ case Rep 15(3)
Rao PS (2023) Advances in the diagnosis and management of congenital heart disease in children. Children 10(4)
Miller PE, Arias S, Lee H, Yarmus L, Feller-Kopman D (2014) Complications associated with the use of the amplatzer device for the management of tracheoesophageal fistula. Annals Am Thorac Soc 11(9):1507–1509
Funding
This work was supported by Open Fund of Hubei Key Laboratory of Tumor microenvironment and Immunotherapy (2022KZL2-02).
Author information
Authors and Affiliations
Contributions
LinTeng secured the funding, Lin Teng and Xinyu Song conceived and designed the study. Lin Teng wrote the main manuscript text, Fei Zhou, Xiaoqi Xiong, Haoyu Zhang, Linchen Qiao, Zaiqiang Zhang, and Qin Qin performed most of the experimental work andformal analysis. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Yichang Central People’s Hospital (Approval No. 2021-026-01).
Informed consent statement
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teng, L., Zhou, F., Xiong, X. et al. Minimally invasive palliative treatment of malignant tracheoesophageal fistula using cardiac septal occluder. Langenbecks Arch Surg 409, 169 (2024). https://doi.org/10.1007/s00423-024-03363-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03363-3