Abstract
Purpose
The purpose of this study was to investigate the impact of autofluorescence technology on postoperative parathyroid function and short-term outcomes in patients undergoing thyroid surgery.
Methods
A total of 546 patients were included in the study, with 287 in the conventional treatment group and 259 in the autofluorescence group. Both groups underwent central lymph node dissection, which is known to affect parathyroid function. Short-term outcomes, including rates of postoperative hypocalcemia and parathyroid dysfunction, serum calcium and PTH levels on the first postoperative day, as well as the need for calcium supplementation, were analyzed. A multivariable analysis was also conducted to assess the impact of autofluorescence on postoperative parathyroid dysfunction, considering factors such as age, BMI, and preoperative calcium levels.
Results
The autofluorescence group demonstrated significantly lower rates of postoperative hypocalcemia and parathyroid dysfunction compared to the conventional treatment group. The autofluorescence group also had better serum calcium and PTH levels on the first postoperative day, and a reduced need for calcium supplementation. Surprisingly, the use of autofluorescence technology did not prolong surgical time; instead, it led to a shorter hospitalization duration. The multivariable analysis showed that autofluorescence significantly reduced the risk of postoperative parathyroid dysfunction, while factors such as age, BMI, and preoperative calcium levels did not show a significant correlation.
Conclusion
This study provides evidence that autofluorescence technology can improve the preservation of parathyroid function during thyroid surgery, leading to better short-term outcomes and reduced postoperative complications. The findings highlight the potential of autofluorescence as a valuable tool in the management of parathyroid hypofunction. Further research and validation are needed to establish the routine use of autofluorescence technology in the thyroid.
Similar content being viewed by others
Introduction
Advancements in global healthcare have facilitated the early diagnosis and timely surgical intervention of numerous thyroid diseases. Postoperative hypoparathyroidism (POHP), considered one of the most common complications following thyroid surgery, primarily results from inadvertent resection or vascular damage during the procedure, leading to acute or chronic disturbances in serum calcium and phosphate metabolism [1,2,3]. Furthermore, it can lead to cardiac injury, long QT syndrome, and cardiomyopathy, causing serious consequences [4]. Additionally, POHP has been linked to neurocognitive disorders, manifesting as seizure-like episodes, cognitive changes, cerebellar, and extrapyramidal symptoms [5]. Therefore, given the escalating global volume of thyroid surgeries, not only does POHP significantly impact the postoperative quality of life for a substantial number of patients, but it also has the potential to contribute to a multitude of cardiovascular, renal, and neurocognitive disorders, posing a grave threat to human health and well-being.
Currently, the main approach to POHP treatment focuses on the symptomatic management of hypocalcemia. However, prolonged and high-dose calcium and vitamin D3 supplementations not only increase the economic burden on patients and adversely affect their quality of life but also significantly disrupt calcium homeostasis, greatly elevating the risk of renal calcification and kidney stone formation [6]. Recombinant human parathyroid hormone (rhPTH) has shown promising results in correcting hypocalcemia, but its widespread application is limited in China due to the need for daily injections and its high cost. Moreover, studies suggest an increased risk of osteosarcoma associated with rhPTH, further constraining its clinical use [7, 8]. Considering the challenges associated with the treatment of hypoparathyroidism, it is crucial to accurately identify and protect the parathyroid glands during thyroid surgery.
Currently, multiple intraoperative imaging methods for parathyroid gland visualization exist. 5-aminolevulinic acid (5-ALA) is a precursor substance of fluorescent dyes that converts into the fluorescent substance protoporphyrin IX (PpIX) within the body [9]. By utilizing specific light sources and fluorescence imaging systems, the fluorescence signal of parathyroid tissue can be observed for visualization. Although it has shown potential imaging effects in some studies, further research and validation are required to ascertain its accuracy and reliability. Additionally, the imaging effect of 5-ALA is influenced by various factors, such as dosage, time, choice of light source, and usage techniques. The uncertainty associated with these factors may impact the consistency and reliability of the imaging results [10, 11]. These factors limit the application of 5-ALA in thyroid surgeries. Nano-carbon and methylene blue are two dyes used for intraoperative negative imaging of parathyroid glands, aiding in their easier identification during surgery [12, 13]. Nano-carbon may generate black deposits in the tissue, somewhat affecting the surgical field. Methylene blue, besides its impact on the surgical field, may experience a weakening of its imaging effect over time, especially during long-duration surgeries, and some patients may exhibit allergic reactions or other adverse effects to it. Therefore, the development of new imaging methods is particularly important.
Parathyroid autofluorescence is a surgical technique that utilizes near-infrared imaging to identify and locate parathyroid glands. Parathyroid tissue naturally emits fluorescence, which can be captured by a near-infrared camera [14, 15]. This technique offers a range of advantages in parathyroidectomy. It reduces the unnecessary removal of parathyroid glands and the associated risk of hypocalcemia by providing more precise localization [16]. Additionally, since it eliminates the need for contrast agents, there is a decreased risk of allergic reactions or adverse effects in patients. Of paramount importance is the real-time feedback it provides, allowing surgeons to observe the fluorescence imaging during the procedure and access immediate information for surgical decision-making [17]. However, due to the relatively new nature of this technology, research on its effectiveness is still limited [18]. This study aims to explore the potential of utilizing parathyroid autofluorescence in thyroid surgery to reduce surgical risks.
Methods
Study design
From September 2021 to January 2023, two surgical experts from Gulou Hospital in Nanjing participated in this study. The surgeons have each performed over 1500 thyroidectomy surgeries annually and have more than 20 years of professional experience. The participants in this study were patients undergoing total thyroidectomy. Exclusion criteria included non-thyroid diseases or previous thyroid surgeries. Patients who did not undergo thyroid surgery, including those admitted with secondary malignant lymph nodes in the neck, were excluded. Patients who were determined to no longer require surgery after evaluation, such as those with subacute thyroiditis, were also excluded. Individuals with a history of both thyroid and parathyroid surgeries were excluded as well. With the help of a computer, patients were randomly assigned to either the standard treatment group (no intraoperative use of autofluorescence) or the autofluorescence group (intraoperative use of autofluorescence). All participants provided informed consent. This study obtained ethical approval from the Ethics Committee of Nanjing Drum Tower Hospital.
Operating procedure
In the conventional treatment group, a meticulous dissection of the thyroid capsule was performed during the thyroidectomy procedure, with careful preservation of the parathyroid glands. In the autofluorescence treatment group, three fluorescence detection processes were carried out during the thyroidectomy procedure. The first detection occurred after opening the thyroid capsule, using the fluorescence system to examine the surgical field and locate each parathyroid gland. Subsequently, the surgery proceeded as per routine. After the thyroid was removed, the second fluorescence detection was performed to ensure the parathyroid glands were preserved in their respective locations. Finally, the third fluorescence detection was conducted on the specimen, assessing for any inadvertently excised parathyroid tissue.
Outcomes
Hypocalcemia is defined as a serum total calcium level below 2.25 mmol/L (normal range, 2.25–2.7 mmol/L) during hospitalization. Postoperative hypocalcemia patients choose oral calcium supplements or a combination of oral calcium supplements and intravenous calcium supplementation based on the severity of their symptoms. In this study, patients did not receive preventive calcium supplementation, meaning that calcium supplementation was not administered if no hypocalcemic symptoms were present after surgery. The collected data included age, gender, BMI index, the extent of central lymph node dissection (not performed, unilateral dissection, bilateral dissection), preoperative calcium level, preoperative parathyroid hormone level, duration of surgery, postoperative calcium level, postoperative day 1 PTH level, a requirement for oral calcium supplementation, a requirement for intravenous calcium supplementation, and inadvertent excision of parathyroid glands in pathology.
Statistical analysis
We analyzed the impact of sample size on the occurrence of parathyroid gland injury in two groups: conventional treatment and autofluorescence treatment in total patients. Parameters assessed included hypocalcemia occurrence, serum PTH levels, length of hospital stay, duration of surgery, and presence of parathyroid glands in pathology. Data analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, USA). A chi-square test was used for categorical variables; a t-test was used for normally distributed continuous variables, and binary logistic regression was used for multivariate analysis, with statistical significance set at p < 0.05.
Results
Baseline characteristics
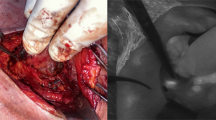
Ultimately, a total of 546 patients were included in this study, with 287 in the control group (conventional treatment) and 259 in the autofluorescence group. In the autofluorescence group, intraoperative fluorescence was used for parathyroid identification (Fig. 1A). Additionally, before specimen submission, fluorescence was employed to check for inadvertently excised parathyroid glands within the specimens (Fig. 1B). All enrolled patients underwent thyroid surgery. Baseline characteristics of patients in both groups were generally well-balanced (Fig. 2). It is worth emphasizing that numerous studies have indicated the significant impact of central lymph node dissection during thyroid surgery on postoperative parathyroid function. Therefore, we placed particular emphasis on comparing the implementation of central lymph node dissection between the two groups and found no significant differences (Table 1).
Intraoperative application of autofluorescence system facilitates postoperative parathyroid preservation. A Intraoperative use of parathyroid autofluorescence system for identification of parathyroid tissue, with green fluorescence indicating parathyroid tissue. B Examination of surgical resection specimens to detect the presence of parathyroid tissue, with green fluorescence indicating inadvertently excised parathyroid tissue during lymph node dissection, necessitating intraoperative autotransplantation
Short-term outcome
An analysis of short-term outcomes for both groups of patients reveals significantly lower rates of postoperative hypocalcemia (p = 0.01) and postoperative parathyroid dysfunction (p < 0.001) in the autofluorescence group compared to the conventional treatment group. Similar results are observed for serum calcium (p = 0.001) and serum PTH (p = 0.023) levels on the first postoperative day. In response to postoperative hypocalcemia symptoms or low postoperative serum calcium levels, a therapeutic calcium supplementation strategy was employed. Upon analyzing postoperative calcium supplementation in both groups, it was evident that the autofluorescence group had significantly lower proportions of patients receiving either oral calcium supplementation (39/259) or intravenous calcium supplementation (34/259) compared to the conventional treatment group receiving oral calcium supplementation (88/287) and intravenous calcium supplementation (76/287). Contrary to our initial expectations, despite using the autofluorescence system to detect the presence of parathyroid glands in the specimens, the final pathology revealed the presence of inadvertently excised parathyroid glands in the autofluorescence group (17/259). However, this proportion was lower than that in the conventional treatment group (40/287). This discrepancy could be attributed to reduced parathyroid damage, and patients utilizing the autofluorescence system experienced a significant reduction in hospitalization duration. Surprisingly, the use of the autofluorescence system did not result in an increase in surgical time, but rather, the conventional treatment group had longer surgical durations (Table 2). To explore the impact of other factors, a multivariable, generalized, linear mixed-effects model was applied to adjust for confounding factors. It was observed that the use of autofluorescence greatly assisted in reducing the risk of postoperative parathyroid dysfunction, while age, BMI, and preoperative calcium levels showed no significant correlation with postoperative parathyroid dysfunction (Table 3).
Autofluorescence impact on central lymph node dissection
Numerous studies have highlighted a strong association between central lymph node dissection and postoperative parathyroid dysfunction. In this study, we conducted a detailed analysis of the data. We categorized the patients into subgroups based on whether central lymph node dissection was not performed, performed on one side, or performed bilaterally. Our results revealed that the use of autofluorescence in patients who did not undergo central lymph node dissection did not significantly affect postoperative serum calcium and PTH levels (Fig. 1C). However, when the autofluorescence system was employed in patients undergoing unilateral central lymph node dissection, although the serum PTH levels did not show statistical differences (p = 0.0572), there was a significant difference in serum calcium levels (p = 0.0333) (Fig. 1D). Remarkably, in patients undergoing bilateral central area clearance, the autofluorescence system exhibited substantial advantages, leading to significant enhancements in both serum calcium levels (p = 0.0012) and PTH levels (p < 0.0001) due to its utilization as shown in Fig. 3C.
Comparison of postoperative serum calcium and PTH levels between the conventional group and the autofluorescence group in different situations. A Comparison of postoperative serum calcium and PTH levels between the conventional group and the autofluorescence group when no central lymph node dissection is performed. B Comparison of postoperative serum calcium and PTH levels between the conventional group and the autofluorescence group when unilateral central lymph node dissection is performed. C Comparison of postoperative serum calcium and PTH levels between the conventional group and the autofluorescence group when bilateral central lymph node dissection is performed
Discussion
The parathyroid glands, four small glands located behind the thyroid, are responsible for regulating calcium and phosphate levels in the body [19, 20]. Accurately identifying and protecting these glands during thyroid or parathyroid surgery is of paramount importance, as their damage or inadvertent removal can lead to severe disruptions in calcium metabolism. We have observed that the use of the autologous fluorescence system, which was developed to precisely and in real-time locate the parathyroid glands [21, 22], can reduce the likelihood of post-thyroid surgery parathyroid gland injuries, thereby lowering the risk of intraoperative misexcision and subsequently reducing the occurrence of postoperative hypocalcemia due to parathyroid gland functional decline [23, 24]. In our study, the utilization of the fluorescence system effectively decreased the probability of parathyroid gland involvement in postoperative pathology, indicating its efficacy in reducing the likelihood of parathyroid misexcision. As previous research reported by Benmiloud et al. [16], the utility of autofluorescence systems in thyroid surgery was confirmed. Considering that most studies support the connection between central lymph node dissection and parathyroid function impairment, we observed the outcomes of applying autofluorescence systems in populations with varying degrees of central lymph node dissection [25]. We categorized patients based on the extent of central lymph node dissection, including those who did not undergo central lymph node dissection, those who had one-side central lymph node dissection, and those who had bilateral central lymph node dissection. Our results demonstrated that the autologous fluorescence system holds greater value for patients requiring bilateral central lymph node dissection. For patients not undergoing central lymph node dissection, the utility of the autologous fluorescence system was not as significant as initially anticipated.
Although parathyroid autofluorescence near-infrared imaging technology has brought numerous benefits to parathyroid surgery, its application still exhibits certain limitations. Firstly, the high equipment cost of near-infrared imaging systems and their associated devices has hindered the adoption of this technology by some healthcare institutions [26,27,28]. While near-infrared imaging can aid physicians in identifying parathyroid glands more easily, there remains a risk of identification errors, particularly in cases involving lesions or abnormal anatomical structures. Additionally, limitations may arise when imaging deep-seated tissues, and there may be shortcomings in displaying parathyroid glands covered by the connective tissue membrane [29, 30]. Currently, the near-infrared system is influenced by the surgical environment, as evident from our intraoperative observations where electrocautery-induced eschar may interfere with fluorescent signals, consequently affecting imaging quality. Despite these limitations and drawbacks, parathyroid autofluorescence near-infrared imaging continues to be considered a valuable tool in parathyroid surgery, especially in complex or high-risk procedures. With ongoing technological advancements and increased clinical research, we can anticipate that these issues will be addressed in the future.
In summary, despite some minor defects in the current autologous fluorescence system, these problems will eventually be overcome with technological progress. Thanks to its non-invasion and excellent performance in parathyroid recognition and protection, we believe that it will become an indispensable and important technology in thyroid surgery in the future.
References
Dedivitis RA, Aires FT, Cernea CR (2017) Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg 25(2):142–146
Orloff LA, Wiseman SM, Bernet VJ et al (2018) American Thyroid Association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 28(7):830–841
Salem FA, Bergenfelz A, Nordenström E, Almquist M (2021) Central lymph node dissection and permanent hypoparathyroidism after total thyroidectomy for papillary thyroid cancer: population-based study. Br J Surg 108(6):684–690
Underbjerg L, Sikjaer T, Rejnmark L (2018) Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: a case-control study. J Bone Miner Res 33(5):822–831
Bohrer T, Krannich JH (2007) Depression as a manifestation of latent chronic hypoparathyroidism. World J Biol Psychiatr 8(1):56–59
Abate EG, Clarke BL (2017) Review of hypoparathyroidism. Front Endocrinol (Lausanne) 7:172
Datsis GA, Berdiaki A, Nikitovic D et al (2011) Parathyroid hormone affects the fibroblast growth factor-proteoglycan signaling axis to regulate osteosarcoma cell migration. FEBS J 278(19):3782–3792
Kuijpers G, Schneider B, Stadel B, Colman E (2002) Recombinant human parathyroid hormone. Preclinical data on rat osteosarcoma were not dismissed. BMJ 324(7347):1218
Takeuchi S, Shimizu K, Shimizu K Jr, Akasu H, Okamura R (2014) Identification of pathological and normal parathyroid tissue by fluorescent labeling with 5-aminolevulinic acid during endocrine neck surgery. J Nippon Med Sch 81(2):84–93
Liu WW, Li CQ, Guo ZM, Li H, Zhang Q, Yang AK (2011) Fluorescence identification of parathyroid glands by aminolevulinic acid hydrochloride in rats. Photomed Laser Surg 29(9):635–638
Asher SA, Peters GE, Pehler SF, Zinn K, Newman JR, Rosenthal EL (2008) Fluorescent detection of rat parathyroid glands via 5-aminolevulinic acid. Laryngoscope 118(6):1014–1018
Ye Z, Wu K, Hu Z, Jin F (2022) Nanocarbon or indocyanine green: which is superior for gasless transaxillary endoscopic thyroidectomy to protect the parathyroid gland? Front Surg 9:1035840
Liu F, Zhu Y, Qian Y, Zhang J, Zhang Y, Zhang Y (2017) Recognition of sentinel lymph nodes in patients with papillary thyroid cancer by nano-carbon and methylene blue. Pak J Med Sci 33(6):1485–1489
Van Slycke S, Van Den Heede K, Brusselaers N, Vermeersch H (2021) Feasibility of autofluorescence for parathyroid glands during thyroid surgery and the risk of hypocalcemia: first results in belgium and review of the literature. Surg Innov 28(4):409–418
Di Marco AN, Palazzo FF (2020) Near-infrared autofluorescence in thyroid and parathyroid surgery. Gland Surg 9(Suppl 2):S136–S146
Benmiloud F, Godiris-Petit G, Gras R et al (2020) Association of autofluorescence-based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: results of the PARAFLUO multicenter randomized clinical trial. JAMA Surg 155(2):106–112
Kose E, Rudin AV, Kahramangil B et al (2020) Autofluorescence imaging of parathyroid glands: an assessment of potential indications. Surgery 167(1):173–179
Solórzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A (2020) Detecting the near infrared autofluorescence of the human parathyroid: hype or opportunity? Ann Surg 272(6):973–985
Tjahjono R, Nguyen K, Phung D, Riffat F, Palme CE (2021) Methods of identification of parathyroid glands in thyroid surgery: a literature review. ANZ J Surg 91(9):1711–1716
Guilmette J, Sadow PM (2019) Parathyroid pathology. Surg Pathol Clin 12(4):1007–1019
Ladurner R, Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ (2019) Parathyroid autofluorescence-how does it affect parathyroid and thyroid surgery? A 5 year experience. Molecules 24(14):2560
Ali KM, Wolfe SA, Nagururu NV et al (2023) Parathyroid gland detection using an intraoperative autofluorescence handheld imager - early feasibility study. Front Endocrinol (Lausanne) 14:1190282
Kim DH, Lee S, Jung J, Kim S, Kim SW, Hwang SH (2022) Near-infrared autofluorescence-based parathyroid glands identification in the thyroidectomy or parathyroidectomy: a systematic review and meta-analysis. Langenbecks Arch Surg 407(2):491–499
Ladurner R, Sommerey S, Arabi NA, Hallfeldt KKJ, Stepp H, Gallwas JKS (2017) Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 31(8):3140–3145
El Khatib Z, Lamblin J, Aubert S et al (2010) Is thymectomy worthwhile in central lymph node dissection for differentiated thyroid cancer? World J Surg. 34(6):1181–1186
Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R (2016) Cutting edge in thyroid surgery: autofluorescence of parathyroid glands. J Am Coll Surg 223(2):374–380
Berber E, Akbulut S (2021) Can near-infrared autofluorescence imaging be used for intraoperative confirmation of parathyroid tissue? J Surg Oncol 124(7):1008–1013
Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A (2011) Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16(6):067012
Su-Velez BM, Hartman GE, Seeley H, Orloff LA, Noel JE, Meister KD (2023) Parathyroid autofluorescence in pediatric thyroid surgery: experience with false positive and false negative results. Otolaryngol Head Neck Surg 169(1):185–189
McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A (2016) Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159(1):193–202
Author information
Authors and Affiliations
Contributions
XianBiao Shi: study conception and design, analysis and interpretation of data, and drafting of manuscript. Guan Lv: acquisition, analysis, and interpretation of data and critical revision of the manuscript. JiaBo Qin: acquisition, analysis, and interpretation of data. HaoRan Ding: acquisition, analysis, and interpretation of data. YiXuan Li: acquisition of data and drafting of the manuscript. LuLu Zheng: acquisition of data. JianFeng Sang: study conception and design and critical revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
JianFeng Sang and Haoran Ding are both corresponding authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, X., Lv, G., Qin, J. et al. The application of autofluorescence system contributes to the preservation of parathyroid function during thyroid surgery. Langenbecks Arch Surg 409, 96 (2024). https://doi.org/10.1007/s00423-024-03256-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03256-5