Abstract
Purpose
Synchronous and metachronous presentations of achalasia and obesity are increasingly common. There is limited data to guide the combined or staged surgical approaches to these conditions.
Methods
A systematic review (MEDLINE, Embase, and Web of Science) and patient-level meta-analysis of published cases were performed to examine the most effective surgical approach for patients with synchronous or metachronous presentations of achalasia and obesity.
Results
Thirty-three studies with 93 patients were reviewed. Eighteen patients underwent concurrent achalasia and bariatric surgery, with the most common (n = 12, 72.2%) being laparoscopic Heller’s myotomy (LHM) and Roux-en-Y gastric bypass (RYGB). This combination achieved 68.9% excess weight loss and 100% remission of achalasia (mean follow-up: 3 years). Seven (6 RYGB, 1 biliopancreatic diversion) patients had bariatric surgery following achalasia surgery. Of these, all 6 RYGBs had satisfactory bariatric outcomes, with complete remission of their achalasia (mean follow-up: 1.8 years). Sixty-eight patients underwent myotomy following bariatric surgery; the majority (n = 55, 80.9%) were following RYGB. In this scenario, per-oral endoscopic myotomy (POEM) achieved higher treatment success than LHM (n = 33 of 35, 94.3% vs. n = 14 of 20, 70.0%, p = 0.021). Moreover, conversion to RYGB following a restrictive bariatric procedure during achalasia surgery was also associated with higher achalasia treatment success.
Conclusion
In patients with concurrent achalasia and obesity, LHM and RYGB achieved good outcomes for both pathologies. For those with weight gain post-achalasia surgery, RYGB provided satisfactory weight loss, without adversely affecting achalasia symptoms. For those with achalasia after bariatric surgery, POEM and conversion to RYGB produced greater treatment success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While achalasia and obesity uncommonly co-occur [1], there is increasing epidemiological evidence linking these two conditions [2,3,4]. Firstly, achalasia is more prevalent in the surgery-naive obese population than in the general population [5,6,7]. Secondly, obesity may manifest following interventions for achalasia [8, 9]. Thirdly, bariatric surgery appears to be a risk factor for post-operative achalasia [10,11,12,13]. Finally, the rising incidence of obesity and associated bariatric procedures suggest that the phenomena of achalasia and obesity will become increasingly prevalent [14]. The complex interplay between these two pathologies and their associated treatment options highlights the need to consider both simultaneously when planning surgery for either.
The aims of surgical treatment for achalasia and obesity are to improve quality of life, alleviate symptoms, prevent long-term sequalae, promote healthy weight loss, and reduce obesity-related co-morbidities. Currently, there is a paucity of data to guide the optimal surgical approach to synchronous or metachronous presentations of achalasia and obesity. This is largely owing to the low prevalence of both conditions in clinical practice. Nonetheless, commentaries from Wesp et al. and Shenfine drew the conclusion that simultaneous procedures for both pathologies were feasible [3, 4]. Additionally, the management of achalasia following Roux-en-Y gastric bypass (RYGB) was explored by Aiolfi et al.’s, which supported either laparoscopic Heller’s myotomy (LHM) or per-oral endoscopic myotomy (POEM) [11].
Despite the above narratives, a systematic appraisal of available evidence for the management of achalasia and obesity has not been undertaken. Accordingly, we performed a systematic review and patient-level meta-analysis of published literature to help guide decision-making around combined (for the synchronous presentation of achalasia and obesity) or staged (for the metachronous presentation of achalasia after obesity surgery or obesity after achalasia surgery) surgical approaches to achalasia and obesity.
Methods
Study identification and screening process
We performed a systematic review and patient-level meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. We conducted a comprehensive search of three databases (MEDLINE, EMBASE, and Web of Science) to identify eligible studies published between 1 January 2000 and 18 July 2022. We used search terms related to achalasia and obesity surgery (Supplementary Table S1). Our search was not language restricted. Furthermore, the bibliographies of each publication were manually reviewed for studies that were not captured in the initial search. References were imported into EndNote™ X8 (Clarivate, Philadelphia, PA, USA), and duplicates were removed. These were then manually screened by two authors (HA and CK) by title and abstract for inclusion. Studies which satisfied the initial screen underwent full-text review. Any uncertainties were resolved by a third author (DL).
Eligibility criteria
Studies selected for final review included patients who underwent surgery (endoscopic, laparoscopic, or open) for obesity and achalasia performed either concurrently or in a staged approach. We included all publications (case reports, case series, and cohort studies) that reported on outcomes of weight loss or achalasia treatment.
Outcomes and definitions
The Eckardt score is a 4-item scale measuring the extent of (1) weight loss in kg, (2) chest pain, (3) regurgitation, and (4) dysphagia for patients with achalasia. Each item is graded on a score of 0–3, with a maximum score of 12. Typically, scores > 3 are suggestive of active achalasia [1]. Accordingly, treatment success for achalasia was defined in this study as an Eckardt score ≤ 3, improvement in Eckardt score ≥ 1 when baseline score < 3 or symptomatic improvement with no re-intervention [1]. Treatment failure for achalasia was defined as those who did not meet the above criteria and had persisting or relapsed symptoms (dysphagia, regurgitation, and chest pain) at follow-up. Weight loss outcomes were defined as percentage excess weight loss (%EWL), percentage body mass index loss (%BMIL), or percentage total body weight loss (%TBWL).
Individual patient data extraction
As all eligible publications were either case reports or case series describing individual cases, we were able to extract individual patient data with sufficient levels of data accuracy and completeness. Where there were missing data, as documented in Table 2 and Supplementary Tables S3 and S4, we attempted to contact the original investigators for clarification. However, due to a lack of response, these data points remained missing. Data was extracted independently by two authors (HA and CK) using a standardized electronic proforma. Patient-level meta-analysis was performed by two other authors (DL and SK). Study characteristics extracted included journal, author, year published, country of origin, study design, and number of patients. Patient characteristics extracted included age, gender, body mass index (BMI), achalasia subtype, previous obesity or achalasia treatment, time between achalasia and obesity surgeries, as well as admission data including length of stay, operation type, myotomy length, post-operative complications, follow-up duration, and treatment outcomes.
Assessment of bias, methodological quality, and synthesis
We employed the proposed methods and tool by Murad et al. to assess for the presence of bias (Supplementary Table S2), methodological quality, and synthesis of available case reports and case series which contributed to this patient-level meta-analysis.
Data analysis
Analyses were performed using Prism v9 (GraphPad Software, San Diego, CA, USA). Study and patient-level data were summarized using descriptive statistics. Patient-level meta-analysis was performed, comparing categorical variables using Fisher’s exact test, and continuous variables using Student’s t-test. Statistical significance was defined as a two-tailed p < 0.05 and 95% confidence interval (CI) around the odds ratio (OR) that did not cross one.
Results
Study selection and characteristics
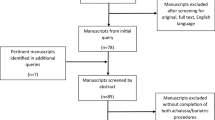
Our systematic review identified a total of 894 studies (Fig. 1). Following screening, 33 were included in the final meta-analysis. All 33 publications were either case reports or case series that described in detail the history, investigations, treatments, and outcomes of 93 patients. As a result, a patient-level meta-analysis using descriptive and comparative statistics was performed. The assessment of potential bias, methodological quality, and synthesis for these case reports and case series are detailed in Supplementary Table S2. All included studies reached a high level of quality for case ascertainment, causality, and reporting. Some deficiencies were noted in case selection as it was unclear in some studies whether the reported case represented the whole experience of that center.
Concurrent surgery for obesity and achalasia
In total, 18 patients were identified from 10 studies (Table 1 and Supplementary Table S3) [16,17,18,19,20,21,22,23,24,25], with a mean (SD) age of 47.9 (14.5) years. Of these, 12 were female, with a mean (SD) body mass index (BMI) of 45.9 (6.7) kg/m2. Four patients had prior pneumatic dilatation for their achalasia, one of whom also received a botox injection (Supplementary Table S3). Overall, thirteen (72.2%) patients underwent concurrent LHM and RYGB. At a mean (SD) follow-up of 27.5 (23.9) months, 12 patients (data was unavailable for 1 patient) achieved satisfactory weight loss, and all had remission of their achalasia symptoms (Table 1). There are potential pitfalls and technical considerations when combining LHM with RYGB [3, 4, 11]. A myotomy first approach allows any mucosal perforation to be easily repaired and covered with a fundoplication. The surgeon then has the option of deferring the RYGB to another day. Alternatively, if the RYGB has already been created, the gastric remnant may be used to cover the site of mucosal perforation. Secondly, during the creation of the gastric pouch for an RYGB, the passage of an endoscope under vision rather than blindly with a bougie may reduce the risk of inadvertent perforation of the myotomized esophagus. Thirdly, when fashioning the gastro-jejunostomy of an RYGB, care must be taken to avoid stapling across the myotomized stomach to minimize the risk of anastomotic leakage.
Surgery for obesity following achalasia surgery
Seven patients were identified from 3 studies (Table 2) [26,27,28], with a mean (SD) age of 40.6 (6.2) years. All patients had a prior myotomy (4 LHM, 3 POEM), with a mean (SD) time from myotomy to bariatric surgery of 16.1 (20.2) months. Of these, 6 (85.7%) patients underwent RYGB for obesity. At a mean (SD) follow-up of 21.2 (12.1) months, their mean (SD) %TBWL was 30.9 (14.4) %, and all 6 patients remained in achalasia remission at follow-up.
Surgery for achalasia following bariatric surgery
Overall, 68 patients were identified from 23 studies (Table 3 and Supplementary Table S4) [11, 16, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47], with a mean (SD) age of 51.8 (9.3) years. Of these, 47 (69.1%) were female, with a mean (SD) BMI of 36.5 (11.1) kg/m2. All achalasia cases were presented after bariatric surgery and were diagnosed using high-resolution or conventional manometry. Overall, 29 (42.6%) patients received prior endoscopic interventions for their achalasia, as detailed in Supplementary Table S4. The mean (SD) time from bariatric to achalasia surgery was 7.2 (3.5) years. Fifty-five (80.9%) patients had a prior RYGB, and 13 (19.1%) had previously undergone a restrictive bariatric procedure (9 sleeve gastrectomies [SG], 2 vertical band gastroplasties [VBG], and 2 duodenal switches [DS]). Of those with a prior RYGB, 35 (63.6%) underwent POEM for definitive treatment of achalasia, while 20 (36.4%) received a Heller’s myotomy. Of those with an underlying restrictive gastric anatomy, all 13 patients received a myotomy; however, 3 patients also underwent conversion to RYGB.
We compared the characteristics of patients who achieved treatment success following achalasia surgery versus those with treatment failure (Table 3). These two groups were comparable in their year of publication, country of origin, follow-up duration, age, BMI, previous achalasia treatments, mix of achalasia subtypes, time from bariatric to achalasia surgery, myotomy length, length of stay, and post-operative complications following achalasia surgery. We found that female gender was associated with treatment success (OR 4.20, 95% CI 1.12–14.34, p = 0.037), and prior SG was associated with treatment failure (OR 5.10, 95% CI 1.30–24.37, p = 0.045). Interestingly, of those with a prior RYGB, patients who underwent POEM achieved a significantly higher rate of clinical success than those who received a Heller’s myotomy (94.3% versus 70.0%, OR 7.07, 95% CI 1.42–36.30, p = 0.021). Furthermore, we identified that conversion to RYGB after a previous restrictive bariatric procedure during achalasia surgery was associated with a trend (p = 0.09) toward higher achalasia treatment success (Table 3).
Discussion
In this systematic review and patient-level meta-analysis, we described for the first time the global surgical experience of managing achalasia and obesity.
Concurrent surgery for obesity and achalasia
We found that LHM and RYGB were the most common approaches for concurrent treatment of obesity and achalasia. This may be for several reasons. Firstly, RYGB minimizes post-myotomy gastroesophageal reflux [48]. This is achieved through the creation of a small gastric pouch, exclusion of acid-producing gastric mucosa, decreased intra-gastric pressure, improved gastric drainage, and prevention of bile reflux [3]. Secondly, both procedures utilize the same patient positioning and port placement and thus can be conveniently performed together [3]. Thirdly, given that achalasia predisposes to carcinomas of the esophagus [1], RYGB retains the native stomach for future conduit formation.
Other combinations of bariatric and achalasia surgeries have significant limitations. For example, LHM and single anastomosis gastric bypass predispose to gastroesophageal bile reflux [49]. LHM and SG are synergistically refluxogenic [48, 50]. Additionally, SG precludes the stomach from future reconstruction after an esophagectomy. LHM and gastric banding are inherently counter-productive, as banding will likely reverse the effect of the myotomy. Substituting LHM with POEM is possible, but unnecessarily adds complexity to any bariatric procedure.
Surgery for obesity following achalasia surgery
We found that RYGB was the bariatric procedure of choice for most authors following achalasia surgery. This is likely explained by the advantages described above. In patients who have undergone a POEM procedure, RYGB should be relatively straightforward, given the paucity of adhesions. In contrast, in the author’s experience, we found that RYGB after Heller’s myotomy is technically more challenging due to hiatal adhesions and the need to release the pre-existing fundoplication. For a pre-existing anterior fundoplication, this dissection may predispose to esophageal injury (particularly at the myotomy site) as well as contribute to fundal ischemia (particularly if the short gastric vessels were previously divided). Strategies to mitigate these risks include meticulous dissection, early use of endoscopy to define the myotomy site, and fundal resection following wrap release. If a mucosal breach occurred, the gastric remnant can be used to patch the area in addition to suture repair. We found that posterior fundoplication is less problematic in this regard. Another potential option is to leave the prior myotomy and fundoplication in place and perform the gastric bypass below the fundoplication rather than risk injury to the prior myotomized area during the reversal of the fundoplication.
Surgery for achalasia following bariatric surgery
Our analysis suggests that POEM is more efficacious than Heller’s myotomy after RYGB. This is a novel and interesting finding, particularly given that both cohorts had comparable myotomy lengths. We postulate that perhaps an endoscopic approach may achieve a “more complete” myotomy since it is traversing virgin tissues relative to a transabdominal approach. In addition, identification of anatomical landmarks using an endoscope may be easier than on laparoscopy in this setting, thus facilitating an adequate myotomy. In this regard, we feel that endoscopy is a valuable adjunct to Heller’s approach to confirm the adequacy of the myotomy.
Moreover, our analysis has demonstrated that prior SG is a risk factor for treatment failure following achalasia surgery. Additionally, we found that conversion to RYGB after a previous restrictive bariatric procedure may improve treatment outcomes for achalasia. These findings are supported by studies demonstrating that restrictive bariatric procedures, particularly SG, significantly increase the risk of post-operative dysphagia, regurgitation, and reflux [13, 51,52,53]. These adverse effects may be interpreted by patients as a failure of their achalasia treatment.
We acknowledge several limitations within our analysis. Firstly, our findings are based on case reports and case series. While this has enabled a detailed patient-level meta-analysis, we are limited by a small sample size and publication bias. Nonetheless, due to the relatively low incidence of obesity and achalasia, it would be impractical to conduct prospective studies in this area. Secondly, we are unable to comment on the role of other endoscopic interventions for achalasia (e.g., botox injection and balloon dilatation) in our treatment algorithm. However, it is recognized that, unlike surgery, botox injection and balloon dilatation do not provide durable achalasia remission [54,55,56]. Moreover, this systematic review was intended to focus on surgical options for both achalasia and obesity. In this regard, there is a bias toward higher numbers of RYGB relative to other weight loss procedures, making comparison between different surgical options challenging. Thirdly, our findings need to be interpreted within the follow-up period available to us from each study. Finally, due to limited data, we could not meta-analyze or comment upon preoperative investigations, perioperative complications, and metabolic outcomes related to these surgical approaches.
Conclusion
Our analysis suggests that a combination of LHM and RYGB adequately treats synchronous presentations of achalasia and obesity. For patients with obesity following achalasia surgery, RYGB enables weight loss without adversely affecting achalasia symptoms. For patients who develop achalasia after bariatric surgery, POEM and conversion to RYGB are associated with the highest rate of treatment success for achalasia. Within the context of available evidence and analysis, we propose a flow diagram to help guide decision-making around combined or staged operative approaches to synchronous or metachronous presentations of achalasia and obesity (Fig. 2).
References
Eckardt AJ, Eckardt VF (2009) Current clinical approach to achalasia. World J Gastroenterol: WJG 15(32):3969
Newberry C, Vajravelu RK, Pickett-Blakely O, Falk G, Yang YX, Lynch KL (2018) Achalasia patients are at nutritional risk regardless of presenting weight category. Dig Dis Sci 63(5):1243–1249
Wesp JA, Farrell TM (2018) The treatment of achalasia in obese patients. Am Surg 84(4):501–505
Shenfine J (2020) Obesity and achalasia. Ann Esophagus 3:28. https://doi.org/10.21037/aoe-201
Sadowski D, Ackah F, Jiang B, Svenson L (2010) Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil 22(9):e256–e261
Lemme EMdO, Alvariz AC, Pereira GLC (2021) Esophageal functional disorders in the pre-operatory evaluation of bariatric surgery. Arquivos de Gastroenterologia 58:190–194
Popescu AL, Costache RS, Costache DO, Balaban VD, Jinga M, Ionita-Radu F et al (2021) Manometric changes of the esophagus in morbidly obese patients. Exp Ther Med 21(6):1–5
Perez-Ortiz AC, Narváez-Chávez S, Furuzawa-Carballeda J, Coss-Adame E, Valdovinos-Díaz MA, Peralta-Figueroa J et al (2020) Long-term risk of adult overweight and obesity among achalasia patients who underwent Heller myotomy. Neurogastroenterol Motil 32(10):e13921
Hew S, Motomura D, Bechara R (2021) Changes in weight following peroral endoscopic myotomy: results from a North American center. Endosc Int Open 9(10):E1491–E1496
Shah RN, Izanec JL, Friedel DM, Axelrod P, Parkman HP, Fisher RS (2004) Achalasia presenting after operative and nonoperative trauma. Dig Dis Sci 49(11):1818–1821
Aiolfi A, Tornese S, Bonitta G, Rausa E, Micheletto G, Bona D (2019) Management of esophageal achalasia after Roux-en-Y gastric bypass: narrative review of the literature. Obes Surg 29(5):1632–1637
Miller AT, Matar R, Dayyeh BKA, Beran A, Vela MF, Lacy BE et al (2020) Postobesity surgery esophageal dysfunction: a combined cross-sectional prevalence study and retrospective analysis. Off J Am Coll Gastroenterol| ACG 115(10):1669–1680
Sans A, Frey S, De Montrichard M, Takoudju C, Coron E, Blanchard C (2021) Impact on sleeve gastrectomy in patients with esophageal motor disorder. Surg Obes Relat Dis 17(11):1890–1896
Seidell JC, Halberstadt J (2015) The global burden of obesity and the challenges of prevention. Ann Nutr Metab 66(Suppl. 2):7–12
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1
Almogy G, Anthone GJ, Crookes PF (2003) Achalasia in the context of morbid obesity: a rare but important association. Obes Surg 13(6):896–900
Kaufman JA, Pellegrini CA, Oelschlager BK (2005) Laparoscopic Heller myotomy and Roux-en-Y gastric bypass: a novel operation for the obese patient with achalasia. J Laparoendosc Adv Surg Tech A 15(4):391–395
O’Rourke RW, Jobe BA, Spight DH, Hunter JG (2007) Simultaneous surgical management of achalasia and morbid obesity. Obes Surg 17(4):547–549
Hagen ME, Sedrak M, Wagner OJ, Jacobsen G, Talamini M, Horgan S (2010) Morbid obesity with achalasia: a surgical challenge. Obes Surg 20(10):1456–1458
Leon P, Csendes A, Braghetto I, Lasen D, Robles J (2010) Achalasia in morbidly obese patients. Report of two cases. Revista Chilena De Cirugia 62(2):172–174
Pinto G, Pestana J, Marin VD, Sendrea JG, Obregon F (2010) Achalasia and morbid obesity: simultaneous management by Heller myotomy and gastric bypass. Obes Surg 20(8):1035–1135
Fisichella PM, Orthopoulos G, Holmstrom A, Patti MG (2015) The surgical management of achalasia in the morbid obese patient. J Gastrointest Surg 19(6):1139–1143
Friedman DT, Duffy AJ (2020) Outcomes of routine upper gastrointestinal series screening and surveillance after laparoscopic adjustable gastric banding. Surg Endosc Other Interv Tech 34(5):2178–2183
Ithurralde-Argerich J, Rosner L, Faerberg A, Puma R, Ferro D, Cuenca-Abente F (2021) Laparoscopic Heller Myotomy and Roux-en-Y gastric bypass as treatment for patients with achalasia and morbid obesity: outcomes in a short series of patients. J Laparoendosc Adv Surg Tech A 31(1):29–35
Velotti N, Vitiello A, Berardi G, Musella M (2021) Roux-en-Y gastric bypass and heller myotomy: one-step surgical treatment of symptomatic achalasia in a morbid obese patient. Obes Surg 31(7):3379–3381
Herbella FA, Matone J, Lourenço LG, Del Grande JC (2005) Obesity and symptomatic achalasia. Obes Surg 15(5):713–715
Bashir U, El Abiad R, Gerke H, Keech J, Parekh K, Nau P (2019) Peroral endoscopic myotomy is feasible and safe in a gastric bypass population. Obes Surg 29(11):3523–3526
Crafts TD, Lyo V, Rajdev P, Wood SG (2021) Treatment of achalasia in the bariatric surgery population: a systematic review and single-institution experience. Surg Endosc 35(9):5203–5216
Cho M, Kaidar-Person O, Szomstein S, Rosenthal RJ (2006) Achalasia after vertical banded gastroplasty for morbid obesity: a case report. Surg Laparosc Endosc Percutaneous Tech 16(3):161–164
Ramos AC, Murakami A, Lanzarini EG, Neto MG, Galvão M (2009) Achalasia and laparoscopic gastric bypass. Surg Obes Relat Dis 5(1):132–134
Benavente-Chenhalls LA, Sherman V, Reardon PR (2011) Laparoscopic Heller myotomy and gastric bypass for achalasia after vertical banded gastroplasty. Surg Obes Relat Dis 7(5):664–665
Oh HB, Tang SW, Shabbir A (2014) Laparoscopic Heller’s cardiomyotomy and Roux-en-Y gastric bypass for missed achalasia diagnosed after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 10(5):1002–1004
Yang D, Draganov PV (2014) Peroral endoscopic myotomy (POEM) for achalasia after Roux-en-Y gastric bypass. Endoscopy 46(Suppl 1):E11–E12. UCTN
Torghabeh MH, Afaneh C, Saif T, Dakin GF (2015) Achalasia 5 years following Roux-en-Y gastric bypass. J Minim Access Surg 11(3):203–4
Boules M, Corcelles R, Zelisko A, Batayyah E, Froylich D, Rodriguez J et al (2016) Achalasia after bariatric surgery. J Laparoendosc Adv Surg Tech A 26(6):428–432
Johnson WD, Marshall MB (2016) Surgical management of achalasia in a patient with previous gastric bypass. Innovations (Phila) 11(3):214–216
Masrur M, Gonzalez-Ciccarelli LF, Giulianotti PC (2016) Robotic Heller myotomy for achalasia after laparoscopic Roux-en-Y gastric bypass: a case report and literature review. Surg Obes Relat Dis 12(9):1755–1757
Nguyen D, Dip F, Lo Menzo E, Szomstein S, Rosenthal R (2016) Heller oesophagomyotomy as treatment for achalasia after gastric bypass for morbid obesity. Ann R Coll Surg Engl 98(1):e3-5
Birriel TJ, Claros L, Chaar ME (2017) Laparoscopic Heller myotomy after previous Roux-en-Y gastric bypass. Surg Obes Relat Dis 13(11):1927–1928
Luo RB, Montalvo D, Horgan S (2017) Peroral endoscopic myotomy after gastric bypass: an effective solution for de novo achalasia. Surg Obes Relat Dis 13(2):e1–e3
Aiolfi A, Tornese S, Barbieri L, Panizzo V, Micheletto G, Bona D (2019) Laparoscopic Heller myotomy after Roux-en-Y gastric bypass. Eur Surg-Acta Chirurgica Austriaca 51(4):220–223
Casas MA, Schlottmann F, Herbella FAM, Buxhoeveden R, Patti MG (2019) Esophageal achalasia after Roux-en-Y gastric bypass for morbid obesity. Updat Surg 71(4):631–635
Kim D, Pullat R, Crowley N (2019) Robotic Redo Heller Myotomy after laparoscopic Heller myotomy in a patient with recurrent achalasia after a Roux-en-Y gastric bypass. Am Surg 85(3):e162–e163
Sanaei O, Draganov P, Kunda R, Yang D, Khashab MA (2019) Peroral endoscopic myotomy for the treatment of achalasia patients with Roux-en-Y gastric bypass anatomy. Endoscopy 51(4):342–345
Kolb JM, Jonas D, Funari MP, Hammad H, Menard-Katcher P, Wagh MS (2020) Efficacy and safety of peroral endoscopic myotomy after prior sleeve gastrectomy and gastric bypass surgery. World J Gastrointest Endosc 12(12):532–541
Bomman S, Klair JS, Ashat M, El Abiad R, Gerke H, Keech J, Parekh K, Nau P, Hanada Y, Wong Kee Song LM, Kozarek R, Irani S, Low D, Ross A, Krishnamoorthi R (2021) Outcomes of peroral endoscopic myotomy in patients with achalasia and prior bariatric surgery: A multicenter experience. Dis Esophagus 34(12):doab044. https://doi.org/10.1093/dote/doab044
Donatelli G, Cereatti F, Soprani A (2021) Per oral endoscopic myotomy for the management of achalasia in a patient with prior lap band, sleeve gastrectomy, and Roux-en-Y gastric bypass. Obes Surg 31(6):2843–2844
El-Hadi M, Birch DW, Gill RS, Karmali S (2014) The effect of bariatric surgery on gastroesophageal reflux disease. Can J Surg 57(2):139–144
Keleidari B, Dehkordi MM, Shahraki MS, Ahmadi ZS, Heidari M, Hajian A et al (2021) Bile reflux after one anastomosis gastric bypass surgery: a review study. Ann Med Surg (Lond) 64:102248
Stenard F, Iannelli A (2015) Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol 21(36):10348–10357
Van Rutte P, Smulders J, De Zoete J, Nienhuijs S (2014) Outcome of sleeve gastrectomy as a primary bariatric procedure. J Br Surg 101(6):661–668
Nath A, Yewale S, Tran T, Brebbia JS, Shope TR, Koch TR (2016) Dysphagia after vertical sleeve gastrectomy: evaluation of risk factors and assessment of endoscopic intervention. World J Gastroenterol 22(47):10371
Pavone G, Tartaglia N, Porfido A, Panzera P, Pacilli M, Ambrosi A (2022) The new onset of GERD after sleeve gastrectomy: A systematic review. Ann Med Surg (Lond) 77:103584. https://doi.org/10.1016/j.amsu.2022.103584
Zaninotto G, Annese V, Costantini M, Del Genio A, Costantino M, Epifani M et al (2004) Randomized controlled trial of botulinum toxin versus laparoscopic Heller myotomy for esophageal achalasia. Ann Surg 239(3):364
Nijhuis RAO, Prins LI, Mostafavi N, van Etten-Jamaludin FS, Smout AJ, Bredenoord AJ (2020) Factors associated with achalasia treatment outcomes: systematic review and meta-analysis. Clin Gastroenterol Hepatol 18(7):1442–1453
Soheilipour M, Zirachi D, Bavandipour A et al (2023) Endoscopic balloon dilation versus laparoscopic heller myotomy: comparing two treatment methods for achalasia. Indian J Surg 85:559–564. https://doi.org/10.1007/s12262-022-03521-1
Acknowledgements
We would like to acknowledge the assistance of Dr. Darren Wong, biostatistician, for his help in the assessment of bias, methodological quality, and synthesis of case reports and case series included in this meta-analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Study conception and design: DSL, AA; Acquisition of data: SK, HA, CC; Analysis and interpretation of data: SK, HA, CC, ST, AA, DSL; Drafting of the manuscript: SK, HA, CC, ST, AA, DSL; Critical revision of the manuscript: SK, HA, CC, ST, AA, DSL.
Corresponding author
Ethics declarations
Conflict of interest
The senior author is a paid lecturer for 3 M Science. All other authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kunz, S., Ashraf, H., Klonis, C. et al. Surgical approaches for achalasia and obesity: a systematic review and patient-level meta-analysis. Langenbecks Arch Surg 408, 403 (2023). https://doi.org/10.1007/s00423-023-03143-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03143-5