Abstract
Background
To evaluate recurrence in patients with post-neoadjuvant pathological complete response (pCR) and in patients with complete response of primary tumor but persisting lymphatic spread of disease (non-pCR, ypT0ypN +) of esophageal cancer.
Methods
Seventy-five patients (63 pCR, 12 non-pCR) were analyzed retrospectively. Pattern and incidence of local and distant recurrence as well as the impact on overall (OS) and disease-free survival (DFS) were evaluated. The efficacy of neoadjuvant chemotherapy according to FLOT protocol was compared to neoadjuvant chemoradiation according to CROSS protocol.
Results
In the pCR group, isolated local recurrence was diagnosed in 3%, while no isolated local recurrence was observed in the non-pCR group due to the high incidence of distant recurrence. Distant recurrence was most common in both cohorts (isolated distant recurrence: pCR group 10% to non-pCR group 55%; simultaneous distant and local recurrence: pCR group 3% to non-pCR group 18%). Median time to distant recurrence was 5.5 months, and median time to local recurrence was 8.0 months. Cumulative incidence of distant recurrence (with and without simultaneous local recurrence) was 16% (± 6%) in pCR patients and 79% (± 13%) in non-pCR patients (hazard ratio (HR) 0.123) estimated by Kaplan–Meier method. OS (HR 0.231) and DFS (HR 0.226) were significantly improved in patients with pCR compared to patients with non-pCR. Advantages for FLOT protocol compared to CROSS protocol, especially with regard to distant control of disease (HR 0.278), were observed (OS (HR 0.361), DFS (HR 0.226)).
Conclusion
Distant recurrence is the predominant site of treatment failure in patients with pCR and non-pCR grade 1a regression, whereby recurrence rates are much higher in patients with non-pCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, esophageal cancer (EC) is the 7th most common cancer and accounts for half a million deaths annually [1]. While the incidence of esophageal squamous cell cancer (ESCC) is stable, especially in western countries, the incidence of esophageal adenocarcinoma (EAC) and esophagogastric junction cancer (AEG) is on the rise [2]. Standard treatment options for locally advanced EC are neoadjuvant chemoradiation (nCRT) according to the CROSS protocol (EAC and ESCC) or perioperative/neoadjuvant chemotherapy (nCT) according to FLOT protocol (EAC) [3, 4]. This is typically followed by surgical resection of the tumor either by partial esophagectomy or transhiatal extended gastrectomy. A substantial fraction of patients achieves pathological complete response (pCR) with neoadjuvant treatment and therefore does not exhibit remaining vital tumor cells on postoperative histopathological examination. pCR is diagnosed in 16–35% of EAC patients after nCT and in 48% of ESCC patients after nCRT and correlates with improved overall (OS) and disease-free survival (DFS) [5, 6].
In recent years, the option of organ-preserving treatment by active surveillance and surgery as needed compared to standard surgery on principle in patients with clinical complete response (cCR) after neoadjuvant treatment has come into focus [7, 8]. Active surveillance offers the option of organ preservation, presumably without significantly increasing the rate of uncontrolled local recurrence/regrowth. The key for successful active surveillance seems to be accurate identification and classification of patients with cCR and conformity with pCR. At present, the prospectively evaluated preSANO protocol describes the most in-depth post-neoadjuvant work-up to identify patients with cCR. Comparison of cCR and pCR demonstrated a sensitivity of 74% and specificity of 77%. This leads to a negative predictive value of 45%, and especially, the accurate determination of the N stage is a difficult task [9]. However, active surveillance seems to have no significant impact on distant recurrence compared to current standard treatment options. The distant recurrence rate is mainly determined by the lymph node status of disease after neoadjuvant treatment [6, 8, 10]. Currently, several ongoing and planned trials are trying to generate evidence for future decision-making (SANO (NTR6803), ESOSTRATE (NCT02551458), and ESORES (preliminary registration identifier: DRKS 00022801)). Hence, the assessment of patients with “true” pCR (ypT0ypN0cM0) and those rare patients with complete response of the primary tumor but persisting lymphatic spread of disease (non-pCR, ypT0ypN + cM0) seems to be of utmost importance.
The main goals of our study were therefore to describe and compare the differences in the incidence and pattern of recurrence in patients with pCR and non-pCR, to compare the survival outcomes, and to compare the efficacy of both neoadjuvant treatment options with regard to local and distant control of tumor recurrence.
Methods
Patient selection
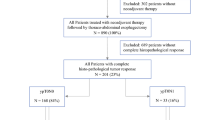
This study is reported in accordance with the STROBE statement [11]. Patient data from 01/2014 to 01/2021 are based on our single-center database for upper gastrointestinal surgery (DRKS 00024369) and evaluated retrospectively. According to the study protocol, all consecutive patients with EC (ESCC and EAC), AEG, and adenocarcinoma with gastric location were screened for this study. Further inclusion criteria were the preoperative administration of either neoadjuvant chemotherapy according to the FLOT protocol [4] or neoadjuvant chemoradiation according to the CROSS protocol [3] and proof of postoperative pathological regression grade 1a (pathological complete response of primary tumor) according to Becker et al. [12] on postoperative histopathological examination. Out of 540 patients with upper gastrointestinal cancer, 393 patients with EC (341 with EAC, 52 with ESCC) were treated with neoadjuvant multimodal treatment in curative intent. Out of these patients, 75 (52 EAC, 23 ESCC) fulfilled all inclusion criteria and were included for all subsequent analyses (Fig. 1). The study was reviewed and approved by the Ethics Committee of the University of Freiburg (21–1093 and 21–1713).
The following variables were extracted from our prospectively maintained database: age, gender, BMI (kg/m2), the Royal College of Surgeons (RCS)-Charlson score [13], pretherapeutic uT stage, pretherapeutic uN stage, and pretherapeutic cM stage according to the American Joint Committee on Cancer/UICC 8th cancer staging manual [14] as well as the postoperative TNM classification, pretherapeutic grading, neoadjuvant treatment modality, the interval between end of neoadjuvant treatment and surgery, type of surgical procedure, and the comprehensive complication index (CCI) [15]. Due to the inclusion criteria, all patients exhibited a ypT0 stage and R0 resection status and had a regression grade 1a according to Becker et al. on postoperative histopathological examination [12]. Furthermore, all patients had a ycM0 situation. Based on negative post-neoadjuvant lymphatic spread, 63 patients (44 EAC and 19 ESCC) were classified as pathological complete response (pCR). Twelve patients (8 EAC, 4 ESCC) with evidence of post-neoadjuvant lymphatic spread (ypN1-3 (ypN +) classification) of disease were classified as non-pathological complete response (non-pCR).
Statistical analysis
Oncologic follow-up was performed according to the German and NCCN guidelines for esophageal cancer treatment [16, 17]. Patients had follow-up computed tomography scans (CT) every 6 months for the first 2 years after surgery and then every 12 months. Based on development of symptoms suspicious for recurrence or post-treatment complications, out-of-routine CT scans as well as endoscopic controls were performed. A total of 42 patients had endoscopic controls. CT scans were available for 63 patients in our radiologic database (DeepUnity Diagnostic 1.1.0.1, Daedalus Healthcare, Bonn, Germany). The patients’ general practitioners or oncologists were contacted to complete the missing follow-up data; the most recent dated November 2022. Median follow-up for disease-free survival (DFS) data was 46 months as estimated by the reversed Kaplan–Meier method [18]. Time and location of recurrence (local, distant) were recorded. Disease-free survival was defined as the interval from surgery to detection of recurrent disease or death from any cause. Local treatment failure was defined as recurrence within the field of surgery. Local treatment failure was further subdivided into endoluminal recurrence, extraluminal/local lymphatic recurrence (supraclavicular recurrence, mediastinal recurrence, or lymphatic recurrence along the vessels of the coeliac trunk), or both. Distant treatment failure was subdivided into distant lymphatic recurrence (outside of the standard D2 lymphadenectomy and the above-mentioned locations), hepatic recurrence, pulmonary recurrence, peritoneal recurrence, recurrence as bone metastases, recurrence as brain metastases (or meningeal carcinomatosis), or recurrence in other sites (one patient with pleural carcinomatosis and one patient with adrenal metastasis). Cumulative incidence of isolated local recurrence and distant recurrence (with or without simultaneous local recurrence) were estimated by the Kaplan–Meier method. Overall survival (OS) data were systematically obtained via the local cancer registry of our Comprehensive Cancer Center Freiburg (CCCF), and follow-up data were collected until November 2022. OS was defined as the interval from surgery to death from any cause. Actuarial survival was calculated by univariate analysis using the Kaplan–Meier method, with log-rank testing for comparison of subgroups. Hazard ratios (HR) were estimated by univariate Cox proportional hazard models. The median follow-up for OS data was 48 months calculated with the reverse Kaplan–Meier method. Results are presented as mean (± standard deviation), median (interquartile range), or as number (percent). We used Mann–Whitney U-test for descriptive analysis for non-parametric variables and Pearson’s chi-squared test for categorical variables. Statistical analysis was performed using SPSS version 28.0.1.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria) with R Studio (R Studio Inc., Boston, MA, USA) and additional packages ggplot2 and survminer. Differences were considered statistically significant when p < 0.05.
Results
Patient characteristics are outlined in Table 1. The median age was 62 years, 54 (72%) of the patients were male, 45 (60%) of patients received nCT according to the FLOT protocol, and 30 (40%) received nCRT according to the CROSS protocol. Treatment-associated 90-day mortality was 3/75 (4%) patients. For the purpose of this study, these patients were excluded from further disease-free survival and site of recurrence analyses, but not from OS analyses.
Pattern of recurrence
The details on the patterns of postoperative recurrence of EC after complete pathological response following neoadjuvant treatment are outlined in Table 2 and supplementary data table S1. Twenty-six events were observed in 18 patients; the recurrence rate was 16% (10/61) in patients with pCR and 73% (8/11) in non-pCR patients.
Recurrence rate was 12% (5/37) in patients with EAC and 26% (5/19) in patients with ESCC following pCR. Most cases of recurrence were distant metastases without local recurrence (pCR group: 6/61 (10%); non-pCR group: 6/11 (55%)). Isolated local recurrence of EC after grade 1a regression was a rare event in both cohorts. In the pCR group, isolated local recurrence was diagnosed in 2 patients (3%), while no isolated local recurrence was observed in the non-pCR group. All cases of local recurrence were local extraluminal/lymphatic recurrences in mediastinal lymph nodes or along the coeliac trunk. No endoluminal recurrences were observed.
Simultaneous distant and local recurrence was observed in 2/61 (3%) in the pCR group and in 2/11 (18%) in the non-pCR group. Regarding the location of distant recurrence, pulmonary recurrence was the most frequently observed site of recurrence in patients with pCR —especially in patients with ESCC (EAC: 1/42; ESCC: 4/19; p = 0.014), while significantly more distant lymphatic recurrences and intracerebral metastases were observed in the non-pCR group.
Timing and pattern of recurrence are shown in the cumulative incidence analysis in Fig. 2. Median time to distant recurrence was 5.5 (3.0–14.5) months, and median time to local recurrence was 8.0 (3.75–21.75) months. In patients with pCR, distant recurrence (with or without simultaneous local recurrence) occurred in 16% (± 6%) of patients, while in non-pCR patients, distant recurrence occurred in 79% (± 13%) of patients following R0 resection of esophageal cancer (HR 0.123 (95% CI: 0.046–0.331, p < 0.001). Isolated local recurrence is a rare event that occurred in 4% (± 2%) of patients with pCR. In the non-pCR group, isolated local recurrence did not occur in our cohort due to the high incidence of simultaneous distant recurrence of disease. This result of the cumulative incidence analysis should not be misinterpreted; 2 of 11 patients (18%) did have distant and local recurrence simultaneously, as shown in Table 2.
Timing of recurrence in patients with pCR and non-pCR after neoadjuvant treatment for esophageal cancer. A Distant recurrence: in patients with pCR, distant recurrence (with or without simultaneous local recurrence) occurred in 16% (± 6%) of patients, while in non-pCR patients, distant recurrence occurred in 79% (± 13%) of patients following R0 resection of esophageal cancer (HR 0.123 (95% CI: 0.046–0.331, p < 0.001). B Isolated local recurrence: isolated local recurrences occurred in 4% (± 2%) of patients with pCR. Isolated local recurrence of EC did not occur in non-pCR patients in our cohort due to the devastating incidence of simultaneous distant recurrence of disease, although 2 of 11 patients (18%) did have simultaneous local recurrence (Table 2)
Survival analysis
OS and DFS were significantly improved in patients with pCR compared to patients with non-pCR. The same observation was made for EAC patients and ESCC patients separately as well (Supplementary data table S2, Figs. S1 and S2).
The EAC patients in our patient cohort received two different types of neoadjuvant treatment — either neoadjuvant/perioperative chemotherapy according to FLOT protocol or neoadjuvant chemoradiation according to CROSS protocol. Based on the underlying database, 45 of 276 (16.3%) patients had grade 1a regression, and 37 of 276 (13.4%) patients had pCR after neoadjuvant chemotherapy according to FLOT protocol. Seven of 45 (15.5%) patients had grade 1a regression and pCR after neoadjuvant chemoradiation according to CROS protocol. The efficacy for the induction of grade 1a regression (p = 0.89) and pCR (p = 0.69) did not significantly differ between the two neoadjuvant treatment protocols. Patients receiving CROSS showed a trend towards being older but did not display higher degree of comorbidities. On the other hand, patients receiving FLOT had a trend towards a higher uN stage and cM stage in the pre-neoadjuvant situation (Table 3). The following observations have to be interpreted in light of this inhomogeneity of the different cohorts. OS and DFS were significantly improved in patients with pCR after nCT according to FLOT protocol compared to nCRT according to CROSS protocol. An improved DFS and a trend towards a better OS following neoadjuvant treatment according to FLOT protocol were also observed in the entire cohort of EAC patients. However, patients with pCR and non-pCR in the FLOT group were compared to only pCR patients in the CROSS group, as all non-pCR patients with EAC were treated with FLOT. Following neoadjuvant treatment according to CROSS protocol, site of failure was more frequently distant recurrence in all EAC patients and in the pCR cohort as well (Table 3, Fig. S3, and Supplementary data tables S3 and S4). The cumulative incidence of distant recurrence (with and without simultaneous local recurrence) was 15% (± 6%) in patients after FLOT compared to 49% (± 20%) following nCRT according to CROSS protocol in all EAC patients (log-rank test: p = 0.050; HR 0.278 (95% CI: 0.071–1.098)). In the pCR cohort of EAC patients (Table 3), the cumulative incidence of distant recurrence (with and without simultaneous local recurrence) was 3% (± 3%) following neoadjuvant treatment according to FLOT protocol, while after neoadjuvant chemoradiation according to CROSS protocol, patients still had distant recurrence in the above-mentioned probability (log-rank test: p < 0.001, HR 0.041 (95% CI: 0.004–0.406)) (Supplementary data Fig. S4). Isolated local recurrence occurred in 0% of patients after FLOT and in 17% (± 15%) in both EAC cohorts cohort after nCRT (p = 0.010 and p = 0.017).
Discussion
In this study, we described the pattern of recurrence in patients with pCR (ypT0ypN0cM0) and non-pCR grade 1a regression (ypT0ypN + cM0) in patients with esophageal cancer after neoadjuvant therapy. Isolated local recurrence was a very rare event in both groups; hence, surgical resection of the primary tumor and systematic lymphadenectomy after neoadjuvant treatment achieves adequate local control of disease. Yet, distant recurrence of esophageal cancer was observed more frequently, in 16% of patients with pCR, and a staggering 79% of cases with non-pCR grade 1a regression.
The distant recurrence was distributed over several locations, with a slight cumulation of pulmonal and cerebral metastases. Patterns of recurrence differ between the two histopathological entities. Location and frequency of recurrence are in line with previous reports of recurrence in patients with pCR [19, 20].
Our study shows a detailed analysis of the recurrence pattern and incidence in patients with non-pCR grade 1a regression. The observed differences in the incidence of recurrence according to UICC stage 0 vs II/III also translated into survival differences in our entire cohort, as shown previously [6, 10, 21]. Due to recent advances in adjuvant treatment after nCRT and the establishment of immunotherapy for EC patients with non-pCR following the results of the CheckMate-577 trial, the survival advantages of pCR patients will probably be attenuated in future [22]. For the establishment of active surveillance protocols, endoluminal and lymph node status are to be assessed for the determination of local cCR. However, our results demonstrate that the main reason for non-pCR grade 1a regression is not inadequate local control of disease but rather distant recurrence. This translates into the overall prognosis of patients with pCR being mainly determined by rapid distant recurrence/progression of disease. Taking current sensitivity and specificity for the detection of persisting local disease based on the preSANO protocol into account, a relevant proportion of patients with non-pCR cannot be identified. Whether these patients profit from performing esophagectomy, which is unlikely to prevent early distant progression of disease, instead of prolonging chemotherapy or combining chemotherapy and immunotherapy has to be elucidated in future studies. With close observation intervals, local progression to irresectable regrowth is unlikely to occur in these patients, and prolonging chemotherapy or administration of immunotherapy is not delayed by surgical resection and possible complications. The diagnostic modalities and efforts to be made to identify lymph node status in patients with complete response of the primary tumor will have to be evaluated in the future, and, in line with other authors, we believe that future research should focus on the identification of biomolecular factors to improve prediction of regrowth or distant recurrence. This would help with the future stratification of patients in active surveillance concepts for clinical complete responders of EC [23, 24]. It is important to keep in mind that standard therapy of EC with pCR is still nCRT/nCT and surgical resection, until high-quality data from the above-mentioned RCTs are made available and present solid evidence for active surveillance. Till then, active surveillance should only be carried out within prospective studies.
Our study shows that in this small sub-cohort of patients with grade 1a regression and pCR from our large cohort of EC patients [6], nCT according to FLOT protocol seems to offer advantages compared to nCRT according to CROSS protocol with regard to overall and disease-free survival. This is especially due to superiority with regard to systemic control of disease. Neither of the two treatment options was superior with regard to induction of pCR in the first place in our study cohort. In contrast to this finding, a recent publication in Annals of Surgery showed that nCRT according to CROSS protocol was associated with a higher rate of pCR (18% vs. 10%) and more R0 resections than nCT according to FLOT protocol, but the postoperative 90-day mortality was higher as well (5% vs 1%), which is mainly attributed to postoperative pulmonary and cardiovascular complications [25]. Data on the comparison of survival differences of the two treatment options from high-quality retrospective studies are rare; results from randomized controlled trials are not yet available. Recent retrospective studies found no survival difference between FLOT and CROSS protocols but better post-neoadjuvant regression after nCRT [26, 27]. To our knowledge, there is a complete lack of data on the comparison of the efficacy of the two treatment options in patients with pCR. Hence, based on the current evidence, no clear recommendation for one of the two neoadjuvant treatment options can be made [28, 29]. Hopefully, with the upcoming results of the ESOPEC trial (NCT02509286), clinicians will have an evidence-based foundation for future clinical decision-making [30]. Possible advantages of nCRT according to CROSS protocol are therefore a higher probability of local tumor control and possibly pCR rate, while the main advantage of perioperative chemotherapy according to FLOT protocol is a better systemic control of disease. It will be interesting to see whether a combination of neoadjuvant radiotherapy and perioperative chemotherapy according to FLOT protocol will combine the above-mentioned possible advantages of both treatment options. This regimen is currently under investigation in the randomized RACE trial (NCT04375605) [31].
Limitations of our study include the retrospective nature as well as the single-center design of the study. Furthermore, the low number of cases, especially in the non-pCR cohort and the CROSS cohort, limits the generalizability of our results. However, we utilize a homogeneous cohort with only two distinct neoadjuvant protocols, either nCT or nCRT, leading to better comparability of treatment groups.
In conclusion, distant recurrence is the predominant site of treatment failure in patients with both pCR and non-pCR grade 1a regression, although recurrence rates are much higher in patients with non-pCR grade 1a regression. Future studies should focus on the improvement of distant control of disease in this cohort of patients.
Data Availability
The datasets and codes generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. https://doi.org/10.3322/caac.21492
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I (2020) Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69(9):1564–1571. https://doi.org/10.1136/gutjnl-2020-321600
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, Van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, Van der Gaast A (2015) CROSS study group Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer. (CROSS) long-term results of a randomised controlled trial. Lancet Oncol 16(9):1090–1098. https://doi.org/10.1016/S1470-2045
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD (2019) FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393(10184):1948–1957. https://doi.org/10.1016/S0140-6736(18)32557-1
Blum Murphy M, Xiao L, Patel VR, DM Maru, Correa AM G, Amlashi F, Liao Z, Komaki R, Lin SH, Skinner HD, Vaporciyan A, Gl Walsh, Swisher SG, Sepesi B, Lee JH, Ms Bhutani, Weston B, Hofstetter WL, Ja Ajani (2017) Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 123(21):4106–4113. https://doi.org/10.1002/cncr.30953
Hipp J, Kuvendjiska J, Hillebrecht HC, Timme-Bronsert S, Fichtner-Feigl S, Hoeppner J, Diener MK (2022) Pathological complete response in multimodal treatment of esophageal cancer: a retrospective cohort study. Dis Esophagus. https://doi.org/10.1093/dote/doac095
Hipp J, Nagavci B, Schmoor C, Meerpohl J, Hoeppner J, Schmucker C (2021) Post-neoadjuvant surveillance and surgery as needed compared with post-neoadjuvant surgery on principle in multimodal treatment for esophageal cancer: a scoping review. Cancers Basel 13(3):429. https://doi.org/10.3390/cancers13030429
van der Wilk BJ, Eyck BM, Hofstetter WL, Ajani JA, Piessen G, Castoro C, Alfieri R, Kim JH, Kim SB, Furlong H, Walsh TN, Nieboer D, Wijnhoven BPL, Lagarde SM, Lanschot JJBV (2022) Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: a systematic review and individual patient data meta-analysis. Ann Surg 275(3):467–476. https://doi.org/10.1097/SLA.0000000000004930
Noordman BJ, Spaander MCW, Valkema R, Wijnhoven BPL, van Berge Henegouwen MI, Shapiro J, Biermann K, van der Gaast A, van Hillegersberg R, Hulshof MCCM, Krishnadath KK, Lagarde SM, Nieuwenhuijzen GAP, Oostenbrug LE, Siersema PD, Schoon EJ, Sosef MN, Steyerberg EW, van Lanschot JJB (2018) SANO study group. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 7:965–974. https://doi.org/10.1016/S1470-2045(18)30201-8
Al-Kaabi A, van der Post RS, van der Werf LR, Wijnhoven BPL, Rosman C, Hulshof MCCM, van Laarhoven HWM, Verhoeven RHA, Siersema PD (2021) Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long-term outcome of esophageal cancer: a population-based study. Acta Oncol 60(4):497–504. https://doi.org/10.1080/0284186X.2020.1870246
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 370(9596):1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X
Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98(7):1521–1530. https://doi.org/10.1002/cncr.11660
Armitage JN, van der Meulen JH (2010) Royal College of Surgeons Co-morbidity Consensus Group Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. . Br J Surg 97(5):772–81. https://doi.org/10.1002/bjs.6930
Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH (2017) Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 67(4):304–317. https://doi.org/10.3322/caac.21399
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7. https://doi.org/10.1097/SLA.0b013e318296c732
Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF (LeitlinienprogrammOnkologie). Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus, Langversion 3.1, 2022, AWMF-Registernummer: 021/023OL. https://www.leitlinienprogramm-onkologie.de/leitlinien/oesophaguskarzinom/. Accessed 2022.11.14
Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA (2019) Esophageal and esophagogastric junction cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 17(7):855–883
Shuster JJ (1991) Median follow-up in clinical trials. J Clin Oncol 9(1):191–192. https://doi.org/10.1200/JCO.1991.9.1.191
de Jongh M, Eyck BM, van der Werf LR, Toxopeus ELA, van Lanschot JJB, Lagarde SM, van der Gaast A, Nuyttens J, Wijnhoven BPL (2021) Pattern of recurrence in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. BJS Open. 5(2):zrab022. https://doi.org/10.1093/bjsopen/zrab022
Barbetta A, Sihag S, Nobel T, Hsu M, Tan KS, Bains M, Jones DR, Molena D (2019) Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J Thorac Cardiovasc Surg. 157(3):1249-1259.e5. https://doi.org/10.1016/j.jtcvs.2018.09.136
Schroeder W, Ghadimi MPH, Schloesser H, Loeser H, Schiller P, Zander T, Gebauer F, Fuchs H, Quaas A, Bruns CJ. (2022) Long-term outcome after histopathological complete response with and without nodal metastases following multimodal treatment of esophageal cancer. Ann Surg Oncol. https://doi.org/10.1245/s10434-022-11700-3
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M (2021) CheckMate 577 Investigators Adjuvant Nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 384(13):1191–1203. https://doi.org/10.1056/NEJMoa2032125
La Mendola R, Bencivenga M, Torroni L, Sacco M, Giacopuzzi S (2021) ASO Author Reflections: Recurrence after pathological complete response in esophageal cancer: analysis of risk factors for this unexpected event. Ann Surg Oncol 28(6):3044–3045. https://doi.org/10.1245/s10434-020-09352-2
Qian DC, Lefferts JA, Zaki BI, Brickley EB, Jackson CR, Andrici J, Sriharan A, Lisovsky M (2022) Development and validation of a molecular tool to predict pathologic complete response in esophageal adenocarcinoma. Dis Esophagus 35(12):doac035. https://doi.org/10.1093/dote/doac035
Kamarajah SK, Griffiths EA. (2022) Oesophago-Gastric Anastomotic Audit (OGAA) Collaborative, Writing group list in the end of manuscript & co-authors listed in the Appendix Postoperative and pathological outcomes of CROSS and FLOT as neoadjuvant therapy for esophageal and junctional adenocarcinoma: an international cohort study from the oesophagogastric anastomosis audit (OGAA). Ann Surg. https://doi.org/10.1097/SLA.0000000000005394
Favi F, Bollschweiler E, Berlth F, Plum P, Hescheler DA, Alakus H, Semrau R, Celik E, Mönig SP, Drebber U, Hölscher AH (2017) Neoadjuvant chemotherapy or chemoradiation for patients with advanced adenocarcinoma of the oesophagus? A propensity score-matched study. Eur J Surg Oncol 43(8):1572–1580. https://doi.org/10.1016/j.ejso.2017.06.003
Donlon NE, Moran B, Kamilli A, Davern M, Sheppard A, King S, Donohoe CL, Lowery M, Cunningham M, Ravi N, Mueller C, Cools-Lartigue J, Ferri L, Reynolds JV (2022) CROSS versus FLOT regimens in esophageal and esophagogastric junction adenocarcinoma: a propensity-matched comparison. Ann Surg 276(5):792–798. https://doi.org/10.1097/SLA.0000000000005617
Babic B, Fuchs HF, Bruns CJ (2020) Neoadjuvante Radiochemotherapie oder Chemotherapie beim lokal fortgeschrittenen Ösophaguskarzinom? [Neoadjuvant chemoradiotherapy or chemotherapy for locally advanced esophageal cancer?]. Chirurg. 91(5):379–383. https://doi.org/10.1007/s00104-020-01150-6
van der Wilk BJ, Eyck BM, Lagarde SM, van der Gaast A, Nuyttens JJME, Wijnhoven BPL, van Lanschot JJB (2019) The optimal neoadjuvant treatment of locally advanced esophageal cancer. J Thorac Dis 11(5):S621–S631. https://doi.org/10.21037/jtd.2018.11.143
Hoeppner J, Lordick F, Brunner T, Glatz T, Bronsert P, Röthling N, Schmoor C, Lorenz D, Ell C, Hopt UT, Siewert JR (2016) ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 19(16):503. https://doi.org/10.1186/s12885-016-2564-y
Lorenzen S, Biederstädt A, Ronellenfitsch U, Reißfelder C, Mönig S, Wenz F, Pauligk C, Walker M, Al-Batran SE, Haller B, Hofheinz RD (2020) RACE-trial: neoadjuvant radiochemotherapy versus chemotherapy for patients with locally advanced, potentially resectable adenocarcinoma of the gastroesophageal junction - a randomized phase III joint study of the AIO, ARO and DGAV. BMC Cancer 20(1):886. https://doi.org/10.1186/s12885-020-07388-x
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study conception and design: J.H., J. Ho., and M.D.; acquisition of data: J.H., S.T., and M.D.; analysis and interpretation of data: J.H. and M.D.; writing—drafting of the manuscript: J.H. and M.D. Writing—critical revision of manuscript: J.H., C.H., J.K., S.H., S.T., S.F.F., J. Ho., and M.D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hipp, J., Kuvendjiska, J., Hillebrecht, H.C. et al. Oncological recurrence following pathological complete response after neoadjuvant treatment in patients with esophageal cancer — a retrospective cohort study. Langenbecks Arch Surg 408, 363 (2023). https://doi.org/10.1007/s00423-023-03100-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03100-2