Abstract
Purpose
Neuropathic pain is a complication after groin hernia surgery. Triple neurectomy of the iliohypogastric nerve, ilioinguinal nerve and genitofemoral nerve is an efficient treatment modality, with several surgical approaches. The minimally invasive endoscopic method to neurectomy was specifically investigated in this meta-analysis. Our aim is to determine the efficacy of this method in the treatment of chronic neuropathic pain posthernia repair surgery.
Methods
A systematic review was conducted using four databases to search for the keywords (“endoscopic retroperitoneal neurectomy” and “laparoscopic retroperitoneal neurectomy”). The NCBI National Library of Medicine, Cochrane Library, MEDLINE Complete and BioMed Central were last searched on 26 May 2022. Randomised control trials and retrospective or prospective papers involving endoscopic retroperitoneal neurectomy operations after inguinal hernia repair were included. All other surgeries, procedures and study designs were excluded. The internal quality of included studies was assessed using the Newcastle–Ottawa Scale. The percentage of patients who had reduction in pain (“positive treatment outcome”) was used to assess the procedure’s effectiveness in each analysis.
Results
Five comparable endoscopic retroperitoneal neurectomy studies with a total of 142 patients were analysed. Both the Wald test (Q (6) = 1.79, = .775) and the probability ratio test (Q (6) = 4.24, = .374) provide similar findings (0.000, 0.0% [0.0%; 78%]). The meta-analysis’ key finding is that the intervention was up to 78% effective (95% confidence interval, 71%; 84%).
Conclusion
Endoscopic retroperitoneal neurectomy can be an effective treatment option for postoperative neuropathic pain relief following surgical hernia repair. Although there is limited reported experience with this technique, it may provide a clinical benefit to the patient. We recommend further prospective data and long-term follow-up studies be conducted to confirm and expand on these outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic pain, defined as pain that lasts for three to 6 months and frequently fluctuates, is a recognized complication in patients after groin hernia repair [1]. In the literature, it has been documented that 0.5–37% of patients experience chronic pain after surgical groin hernia repair [2,3,4,5,6,7].
Chronic neuropathic pain, also known as persistent pain with characteristics like burning or shooting, can occur due to damage to the somatosensory system [1]. Modern open and laparoscopic repairs of groin hernias can cause entrapment-related symptoms from the genital nerves, specifically the genitofemoral and ilioinguinal [4]. Usually, such symptoms are self-limiting within a few weeks following the operation and so require no extended therapy [4]. However, persistent severe symptoms could lead to substantial morbidity and surgical reintervention. It is reported these symptoms are less likely to occur and less likely to be severe using minimally invasive methods [8].
Chronic neuropathic pain significantly influences activity and comfort levels, negatively impacting the patient’s quality of life. It has also been linked to psychiatric illnesses, including anxiety and depression, which may impact a patient’s well-being [9, 10]. Conservative management of neuropathic pain can include pharmacological, interventional and behavioural therapy while surgical options were devised to alleviate recurrent and refractory pain [9, 10]. Triple neurectomy of the ilioinguinal nerve (IIN), iliohypogastric nerve (IHN) and genitofemoral nerve (GFN) has emerged as a viable therapy option for this debilitating pain [3].
Despite the fact that case reports and other retrospective studies exist for open, laparoscopic, retroperitoneal and transperitoneal endoscopic techniques to triple neurectomy [11, 12], this meta-analysis focuses on the least invasive retroperitoneal endoscopic method. Our aim is to determine the efficacy of this method in the treatment of chronic neuropathic pain posthernia repair surgery. To the best of our knowledge, this is the first meta-analysis for this specific minimally invasive surgery.
Anatomy
Endoscopic retroperitoneal neurectomy necessitates a thorough understanding of the IHN, IIN and GFN locations and distributions. The lumbar plexus, represented by T12–L5, gives origin to these three nerves. The IHN, IIN and GFN are attributed to the lumbar plexus and contain parts of T12–L2. All three nerves (Fig. 1) include motor and sensory fibres and pass via the retroperitoneal space. The retroperitoneal space is bounded ventrally by the parietal peritoneum, dorsally by the fascia of the greater psoas muscle, laterally by the transverse fascia and cranially by the diaphragm. Caudally, it continues as the connective tissue of the lesser pelvis [5, 13].
Sensory innervation of the inguinal region, according to https://doctorlib.info/medical/anatomy/29.html
The origins of the IHN are the segments T12–L1. These cords unite to form the IHN and run dorsally to the psoas major muscle on the quadratus lumborum muscle. After penetrating the transversus abdominis muscle, it follows the inguinal ligament medially. Above the superficial inguinal ring, it goes through the external oblique muscle. Its motor fibres innervate the caudal and lateral abdominal muscle, while its cutaneous rami (a branch of a nerve or blood vessel) innervate the skin above the inguinal ligament and the lateral hip region [11, 12, 14, 15].
The IIN is formed by rami anteriores from T12 to L1. The nerve course is analogous to the IHN from the iliac crest to the inguinal ligament medially but breaks through the superficial inguinal ring directly entering the inguinal canal. The motor fibres of the nerve innervate the same areas as those of the IHN. Moreover, it innervates the skin of the penile root or labia majora, with its scrotal or labial anterior nerves [11, 12, 14, 15].
The GNF consists of rami anteriores from the segments L1 and L2. After penetrating the psoas major muscle, it divides into femoral and genital rami. The former travels to the saphenous hiatus by passing through the lacuna vasorum. In men, the genital ramus is part of the spermatic cord in the inguinal canal and innervates the motor portion of the cremasteric reflex and the skin of the scrotum. In women, it supplies the labia majora as it passes through the round ligament. The femoral ramus of the GFN innervates the skin of the medial side of the thigh regardless of gender [11, 12, 14, 15].
Surgical technique of endoscopic retroperitoneal neurectomy
There are many surgical methods allowing for a successful neurectomy of the IHN, IIN and GFN. While analyses of open, laparoscopic transperitoneal and endoscopic procedures for triple neurectomy have been published [16, 17], this meta-analysis focuses on the endoscopic retroperitoneal approach, described in detail in the British Journal of Surgery in 1997 [5].
The process begins with an intravenous antibiotic prophylaxis administered before the surgery. The patient undergoes general anaesthesia and is placed in a supine, slightly lateral posture (Fig. 2). Then, a transverse incision is made midway between the costal border and the anterior superior iliac spine in the midaxillary line. A 12 mm cannula is placed through this incision, and carbon dioxide is insufflated at a pressure of 12 mmHg. Under direct vision, cannulae of 10 mm and 5 mm are placed. Furthermore, a 4–5 cm dissection of the GNF, including the bifurcation, is performed at the level of the psoas muscle. When the IHN/IIN are dissected, the nerves are exposed at the area where they cross the quadratus lumborum muscle and then excised proximally and laterally to the GFN. Furthermore, all resected nerve specimens are subjected to histological examination [18].
Surgical positioning of the patient and access route for endoscopic visualization of the retroperitoneal space. As described in [4, 5, 18, 34]; modified with permission [18]. Left: initial technique described by Krähenbühl et al. [5] for the first time in 1997. Right: modified technique with two 5 mm cannulae and one 10 mm or 12 mm cannula
Methods
Search strategy
The authors A. J., A. T. and D. H. conducted independent searches for literature using the NCBI National Library of Medicine, Cochrane Library, MEDLINE Complete and BioMed Central. The date of the last search was 26 May 2022. The following single-text terms were used in the search strategy: “endoscopic retroperitoneal neurectomy” and “laparoscopic retroperitoneal neurectomy”. The retrieved studies’ reference lists and citations were manually searched for more relevant research.
Inclusion and exclusion criteria
The search technique was based on an orderly structure of core phrases, and only papers involving endoscopic retroperitoneal neurectomy operations after inguinal hernia repair were included. Randomised control trials and retrospective or prospective study designs were included in this study. Exclusion criteria included case reports, case series, comment letters, studies with poor design such as studies with insufficient data and studies with Newcastle–Ottawa quality assessment score of 4 or less [19]. All other surgeries, including open and laparoscopic approaches to neurectomy, as well as any procedures that follow a transperitoneal approach, were excluded.
Data collection
After the removal of duplicates, full-text evaluation and data extraction was completed by two independent authors. The internal quality of included studies was assessed using the Newcastle–Ottawa Scale by two independent authors [20].
Data items and variables
The percentage of patients who had received significant pain reduction postoperatively, in addition to the frequencies and proportions of therapy (“proportion of positive results”), were the primary outcome values assessed in this meta-analysis. Pain reduction was defined as statistically significant pain reduction based on pre- and postoperative questionnaire scores. Questionnaires used in analysed studies include the following: NRS-11: Numeric Rating Scale; I-PROM: Individual Patient-Reported Outcome Measure; NRS: Numerical Rating Scale; PDQ: PainDETECT Questionnaire [3, 4, 8, 18, 21].
Statistical analysis
Statistical analysis was conducted using the R programme Meta (G. Schwarzer, version 4.18–0 from March 5, 2021). The Wald test and the probability ratio test were used to analyse statistical heterogeneity. Furthermore, for the frequencies of positive treatment outcome occurrence, a logistic regression model with a random intercept and a maximum-probability estimator of 2 and the Hartung-Knapp adjustment for random effects models were employed to analyse the outcomes. The model’s outputs were used to calculate the logits of the frequencies’ chances. Due to the fact that the treatments in three separate studies were all positive, the inclusion of the three separate studies in the model was made possible using a continuity correction of 0.5. The findings were accompanied by their 95% Clopper-Pearson confidence intervals. Furthermore, a p < 0.05 was considered statistically significant. In addition, a funnel plot and a radial plot were developed to detect publication bias, and other statistical tests such as a rank correlation test and three regression tests were utilised.
Results
Excluded studies
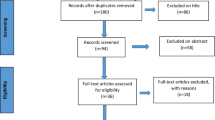
The initial literature search was carried out, with a specific filter set to display full-text articles. As a result, 97 records were discovered, which were reduced to 62 when duplicates were deleted. As a result, 46 papers were omitted because they did not describe surgical procedures involving retroperitoneal surgical access and hence did not match the inclusion criteria. The full text of 16 articles was reviewed. As a consequence, 11 more papers (Table 1) were removed for the following reasons: one for a lack of quality according to the Newcastle–Ottawa Scale [19], three for being comment letters, two for employing an intraabdominal surgical technique, two for being anatomical investigations and one for having just one patient. Two studies were omitted because they contained the same patients as other included research; see Fig. 3.
Study selection
All publications about the minimally invasive endoscopic retroperitoneal approach to inguinal triple neurectomy were included. Five studies were found, with 142 patients of both genders who received successful surgery over a 24-year span. It should also be highlighted that the initial number of patients was 182, but 41 individuals from the five studies were excluded from the analysis, since 8 had persistent pain unrelated to prior hernia surgery and 33 had open neurectomies. These five papers were appropriate for meta-analysis and systematic review (Fig. 4) [3, 4, 8, 18, 21]. The study characteristics are described and summarized in Table 2.
Examination of quality bias
Of the 5 studies included, 100% scored 5 or higher on the Newcastle–Ottawa Scale indicating good quality studies (Table 3) [19].
Systematic review and meta-analysis
The distribution of the findings was highly homogenous (τ2 = 0.000, I2 = 0.0% [0.0%; 70.8%]). The Wald test (Q (6) = 1.79, p = 0.775) and the probability ratio test (Q (6) = 4.24, p = 0.374) did not reveal any significant differences. The meta-analysis was carried out using a fixed effects logistic regression model due to the homogeneity of the research data. Figure 4 shows the frequencies and proportions of treatment and their 95% Clopper-Pearson confidence intervals.
Successful treatment was defined as a significant reduction in pain. The meta-analysis shown concluded that the surgical treatment across all individual studies was 78% successful (95% confidence interval, 71%: 84%) with 111/142 patients experiencing reduced pain. According to the 95% confidence interval, the treatment was successful in at least 71%, meaning in at least two-thirds of cases.
Examination of publication bias
Figure 5 shows a funnel plot which was generated to see if the results may be skewed by a publishing bias. An odds of 1 and hence a 50% success rate for therapy are indicated on the graph, with the x-axis showing logit-transformed proportions. The absence of small studies with non-positive and non-significant outcomes suggests a publishing bias in this compilation.
In Fig. 6, contour lines for several significance levels have been inserted [22]. Non-significant findings do not appear to be concealed in our analysis as is typical in cases of publication bias. There are two small and two major studies with non-significant findings at the 5% level.
Figure 7 depicts a radial map showing the findings of individual investigations [23]. All studies scatter more or less randomly in the 95% confidence interval and around the middle regression line if the treatment effect is 75%, so that the image does not show any red flags for publication bias.
Radial plot for meta-analysis
In addition, four statistical tests were conducted to check the asymmetry of the funnel plot, the results of which are summarized in Table 4. These four tests are the rank correlation test by Begg and Mazumdar [24], the regression test by Egger et al. [25], the regression test by Thompson and Sharp [26] and the regression test by Peters et al. [27]. The tests all lead to the conclusion that the asymmetry of the funnel plot must be viewed as random and that there is no evidence of a publication bias.
Discussion
This systematic review was conducted to characterise the success rate of endoscopic triple neurectomy and thus provide a valid recommendation whether this technique should be considered for patients with postoperative inguinal neuropathic pain. Furthermore, the literature on this technique can be considered valid as thorough statistical evaluation did not reveal any indicators that the collected data might have significant publication bias. Of the 142 patients from five studies who underwent endoscopic triple neurectomy for the indication mentioned above, approximately 75% reported complete or at least significant reduction in subjective pain intensity.
Even though its exact occurrence rate remains controversial, there is consistent evidence that postoperative neuropathic pain has surpassed hernia recurrence as the most frequent complication of hernia repair [28,29,30]. However, surgical denervation after hernia surgery is not indicated in all cases. While most patients with chronic pain can be managed conservatively with medication or therapy, it does not always prove to be straightforward. Patients may have to deal with central nervous system side effects or dependence and still not achieve sufficient pain relief [31].
Triple neurectomy of the IIN, IHN and GFN has been proven to be an excellent treatment method in cases of chronic pain. Stulz and Pfeiffer [32] first described the procedure in 1982 as an ultima ratio method for neuropathic pain caused by nerve entrapment following surgeries in the lower abdomen. Over the years, different surgical techniques for neurectomy were established including open, laparoscopic and endoscopic approaches [10, 18, 33]. Combined procedures, such as open with laparoscopic or open with endoscopic procedure, have been described in the literature [16, 17]. According to several authors, the success rate of these interventions is 70–100% [2]. Only orchialgia does not significantly improve after this intervention, which could be related to the more complex innervation of the gonads [34, 35].
Postoperatively, numbness might be felt over the treated area, a possibility that should be explored and discussed with the patient [34]. A small number of side effects have been described after undergoing these procedures. Among them are loss of cremaster reflex and hypoesthesia in the labia majora, scrotum and skin of the femoral region [34]. However, the significant pain reduction experienced by patients usually outweighs the possible adverse outcomes. The actual frequency of these complications seems to be less than expected. Among 142 patients reviewed in this study, none developed any of the aforementioned complications postoperatively [21]. Triple laparoscopic neurectomy consists of an “open two-stage operation”, which is also performed in patients with chronic groin pain. This method is equally minimally invasive but requires adequate knowledge of the anatomical variations of the genitofemoral nerve and the overall neuroanatomy of the genital and inguinal regions [9]. The success rate of triple laparoscopic neurectomy is much higher than that of the standard procedure with all three nerve branches transected [34].
Another method that may be used if traditional laparoscopic surgery is not feasible is the “dual two-team approach”. It combines open surgical and endoscopic techniques. Although this method showed promise with high success rates on a comparative scale, no definitive conclusion can be drawn due to only one small descriptive study discussing this method [16].
Endoscopic retroperitoneal neurectomy is a one-stage, minimally invasive procedure for resecting the IHN, IIN and GFN from the lumbar plexus. This method has been found to provide excellent pain relief and morbidity rates comparable to open neurectomy, but it is a challenging procedure even in the hands of experts, and anatomical variances make the identification of the nerves even more complex [18].
The retroperitoneal access may present some inconveniences inherent to any surgical procedure, including extensive dissection, painful incision and complications such as surgical wound infection, hematoma, bleeding, incisional hernia and postoperative paralytic ileus. In the population of 142 patients, 3 patients had complications due to the procedure. Two patients had a laceration of the posteroinferior diaphragm requiring laparoscopical suturing and one patient had a delayed haemorrhage requiring transfusion and embolization. No other complications were reported intra- or perioperatively in the population. The advantage of this technique is providing broad access to the retroperitoneal cavity and efficient control of haemorrhage. This procedure is described to be less painful and provides faster postoperative recovery, discharge and earlier return to usual activities [3]. As the subject of this meta-analysis, we evaluated exclusively endoscopic retroperitoneal neurectomy to treat patients with persistent neuropathic pain. Unlike open neurectomy, the endoscopic method proved to be the procedure with the least invasive approach and simultaneously similarly effective in pain relief [18]. While the endoscopic retroperitoneal technique allows visualization of all nerves of the lumbar plexus, the open surgical triple neurectomy provides only identification of the genitofemoral nerve with certainty [18].
There are several limitations to this study. First, this review included articles that contained various methods for quantifying chronic neuropathic pain in posthernia repair surgery. Second, the number of patients analysed is small due to the paucity of published literature on this specific approach which will increase the number of type II errors. Third, the total length of follow-up in the studies included was between 3 months and 1 year. Longer follow-up in future studies will help better understand long-term quality-of-life assessments postprocedure. Large, multicentered prospective and randomised control studies using standardized treatments and well-defined pain outcome measurements must be performed in order to increase the predictive power of postoperative outcomes. This surgical technique is the last line of treatment meaning there can be a learning curve and reduced feasibility in practical application [3]. Our analysis, along with future research, should drive a standardized protocol that can improve both the reproducibility and feasibility of such a method.
Conclusion
Persistent neuropathic pain is a common complication of surgical hernia repair. However, it is often debilitating as it permanently affects the patient’s physical abilities and mental health. As a result, several techniques have been developed to relieve the symptoms of postoperative inguinal neuropathic pain. We conducted a meta-analysis of five studies on the minimally invasive endoscopic retroperitoneal method for ilioinguinal, iliohypogastric and genitofemoral nerve triple neurectomy. Our analysis showed that endoscopic retroperitoneal neurectomy can be an effective treatment option for postoperative neuropathic pain relief following surgical hernia repair. Considering the small number of patients in this study, data interpretation with regard to efficacy can only be limited. Although there is limited reported experience with this technique, it may provide a clinical benefit to the patient. Further prospective data and long-term follow-up of the triple neurectomy procedure will be needed to confirm these outcomes.
Change history
16 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00423-023-02824-5
References
Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R et al (2015) A classification of chronic pain for ICD-11. Pain 156:1003–1007. https://doi.org/10.1097/j.pain.0000000000000160
Ducic I, West J, Maxted W (2008) Management of chronic postoperative groin pain. Ann Plast Surg 60:294–298. https://doi.org/10.1097/sap.0b013e3180de600e
Moore AM, Bjurstrom MF, Hiatt JR, Amid PK, Chen DC (2016) Efficacy of retroperitoneal triple neurectomy for refractory neuropathic inguinodynia. Am J Surg 212:1126–1132. https://doi.org/10.1016/j.amjsurg.2016.09.012
Muto CM, Pedana N, Scarpelli S, Galardo R, Guida G, Schiavone V (2005) Inguinal neurectomy for nerve entrapment after open/laparoscopic hernia repair using retroperitoneal endoscopic approach. Surg Endosc 19:974–976. https://doi.org/10.1007/s00464-004-2007-8
Krähenbühl L, Striffeler H, Baer HU, Büchler MW (1997) Retroperitoneal endoscopic neurectomy for nerve entrapment after hernia repair. Br J Surg 84:216–219
Charalambous MP, Charalambous CP (2018) Incidence of chronic groin pain following open mesh inguinal hernia repair, and effect of elective division of the ilioinguinal nerve: meta-analysis of randomized controlled trials. Hernia 22(3):401–409. https://doi.org/10.1007/s10029-018-1753-9
van Steensel S, van Vugt LK, Al Omar AK, Mommers EHH, Breukink SO, Stassen LPS, Winkens B, Bouvy ND (2019) Meta-analysis of postoperative pain using non-sutured or sutured single-layer open mesh repair for inguinal hernia. BJS Open 3(3):260–273. https://doi.org/10.1002/bjs5.50139
Pedersen KF, Chen DC, Kehlet H, Stadeager MW, Bisgaard T (2021) A Simplified clinical algorithm for standardized surgical treatment of chronic pain after inguinal hernia repair: a quality assessment study. Scand J Surg 110(3):359–367. https://doi.org/10.1177/1457496920954570
Song JW, Wolf JS, McGillicuddy JE, Bhangoo S, Yang LJ-S (2011) Laparoscopic triple neurectomy for intractable groin pain: technical report of 3 cases. Neurosurgery 68:339–346. https://doi.org/10.1227/neu.0b013e3182114480. (discussion 346)
Ramshaw B, Vetrano V, Jagadish M, Forman B, Heidel E, Mancini M (2017) Laparoscopic approach for the treatment of chronic groin pain after inguinal hernia repair: laparoscopic approach for inguinodynia. Surg Endosc 31:5267–5274. https://doi.org/10.1007/s00464-017-5600-3
Drenckhahn D, Christ B, Benninghoff A (eds) (2003) Zellen- und Gewebelehre, Entwicklungslehre, Skelett- und Muskelsystem, Atemsystem, Verdauungssystem, Harn- und Genitalsystem, 16th edn. Urban & Fischer, Anatomie, Bd. 1. München
Schünke M, Schulte E, Schumacher U (2014) PROMETHEUS Allgemeine Anatomie und Bewegungssystem, 4th edn. Georg Thieme Verlag, Stuttgart
Weill FS, Coche G, Costaz R, Didier D, Mouel A, Rohmer P (1990) CT-Fibel Thorax und Abdomen. Springer, Berlin Heidelberg, Berlin, Heidelberg
Zilles K, Tillmann B (2010) Anatomie. Springer-Lehrbuch. Springer-Verlag, Berlin Heidelberg, Berlin, Heidelberg
Benninghoff A, Drenckhahn D, Waschke J, Asan E (eds) (2008) Taschenbuch Anatomie, 1st edn. Elsevier Urban & Fischer, München
George T, Williams EH, Franklin R, Lee DA (2019) Two-team surgical approach to improve retroperitoneal nerve identification in the treatment of groin pain. Ann Plast Surg 82:82–84. https://doi.org/10.1097/sap.0000000000001662
Keller JE, Stefanidis D, Dolce CJ, Iannitti DA, Kercher KW, Heniford BT (2008) Combined open and laparoscopic approach to chronic pain after inguinal hernia repair. Am Surg 74:695–700 (discussion 700–701)
Giger U, Wente MN, Büchler MW, Krähenbühl S, Lerut J, Krähenbühl L (2009) Endoscopic retroperitoneal neurectomy for chronic pain after groin surgery. Br J Surg 96:1076–1081. https://doi.org/10.1002/bjs.6623
Luchini C, Stubbs B, Solmi M, Veronese N (2017) Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Analysis 5(4):80. https://doi.org/10.13105/wjma.v5.i4.80
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2019) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ohri.ca. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Bjurström MF, Nicol AL, Amid PK, Lee CH, Ferrante FM, Chen DC (2017) Neurophysiological and clinical effects of laparoscopic retroperitoneal triple neurectomy in patients with refractory postherniorrhaphy neuropathic inguinodynia. Pain Pract Off J World Inst Pain 17:447–459. https://doi.org/10.1111/papr.12468
Peters JL, Sutton AJ, Jones DJ, Abrams KR, Rushton L (2008) Contour-enhanced metaanalysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61:991–996
Galbraith RF (1988) Graphical display of estimates having differing standard errors. Technometrics 30:271–281
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101. https://doi.org/10.2307/2533446
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Thompson SG, Sharp SJ (1999) Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 18:2693–2708. https://doi.org/10.1002/(sici)1097-0258(19991030)18:20%3c2693::aid-sim235%3e3.0.co;2-v
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295:676–680
Loos MJA, Roumen RMH, Scheltinga MRM (2007) Chronic sequelae of common elective groin hernia repair. Hernia 11(2):169–173. https://doi.org/10.1007/s10029-007-0196-5
Lange JFM, Kaufmann R, Wijsmuller AR, Pierie JPEN, Ploeg RJ, Chen DC, Amid PK (2014) An international consensus algorithm for management of chronic postoperative inguinal pain. Hernia 19(1):33–43. https://doi.org/10.1007/s10029-014-1292-y
Chen D, Bjurstrom M, Amid P, Nicol A (2014) Pain control following inguinal herniorrhaphy: current perspectives. J Pain Res 7:277–90. https://doi.org/10.2147/jpr.s47005
Bonnet U, Scherbaum N (2018) Über das Abhängigkeitspotential von Gabapentinoiden [On the risk of dependence of gabapentinoids]. Fortschr Neurol Psychiatr. 86(2):82–105. https://doi.org/10.1055/s-0043-122392. (German)
Stulz P, Pfeiffer KM (1982) Peripheral nerve injuries resulting from common surgical procedures in the lower portion of the abdomen. Arch Surg (Chicago, Ill.: 1960) 117:324–327. https://doi.org/10.1001/archsurg.1982.01380270042009
Loos MJ, Scheltinga MR, Roumen RM (2010) Tailored neurectomy for treatment of postherniorrhaphy inguinal neuralgia. Surgery 147:275–281. https://doi.org/10.1016/j.surg.2009.08.008
Chen DC, Hiatt JR, Amid PK (2013) Operative management of refractory neuropathic inguinodynia by a laparoscopic retroperitoneal approach. JAMA Surg 148:962–967. https://doi.org/10.1001/jamasurg.2013.3189
Gerendai I, Banczerowski P, Halász B (2005) Functional significance of the innervation of the gonads. Endocrine 28:309–318. https://doi.org/10.1385/endo:28:3:309
Funding
Open access funding provided by University of Basel
Author information
Authors and Affiliations
Contributions
Study conception and design A. T.; acquisition of data, analysis and interpretation of data A. T., A. J., D. H., L. K.; drafting of manuscript A. T., S. T.-M., D. H.; critical revision of manuscript M. Z., V. O., A. T., L. K., A. J.; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taha-Mehlitz, S., Taha, A., Janzen, A. et al. Is pain control for chronic neuropathic pain after inguinal hernia repair using endoscopic retroperitoneal neurectomy effective? A meta-analysis of 142 patients from 1995 to 2022. Langenbecks Arch Surg 408, 39 (2023). https://doi.org/10.1007/s00423-022-02748-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-022-02748-6