Abstract
Purpose
Fast-track protocols are increasingly used after digestive surgery. After esophagectomy, the gravity and the fear of anastomotic leak may be an obstacle to generalization of such protocols. C-reactive protein (CRP) might be a reliable tool to identify patients at low risk of anastomotic leak after esophagectomy, so that they can be safely included in a fast-track program. The aim of our retrospective bicentric study is to evaluate the interest of C-reactive protein measurement for the early diagnosis of anastomotic leak after esophagectomy.
Methods
Patients having undergone Ivor-Lewis procedure between January 2009 and September 2017 were included in this bicentric retrospective study. CRP values were recorded between postoperative day 3 (POD 3) and postoperative day 5 (POD 5). All postoperative complications were recorded, and the primary endpoint was anastomotic leak.
Results
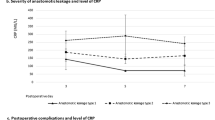
We included 585 patients. Among them, 241 (41.2%) developed infectious complications and 69 patients (11.8%) developed anastomotic leak. CRP had the best predictive value on POD 5 (AUC = 0.74; 95% CI: 0.67–0.81). On POD 5, a cut-off value of 130 mg/L yielded a sensitivity of 87%, a specificity of 51%, and a negative predictive value of 96% for the detection of anastomotic leak.
Conclusions
CRP may help in identifying patients at very low risk of anastomotic leak after esophagectomy. Patients with CRP values < 130 mg/L on POD 5 can safely undertake an enhanced recovery protocol.




Similar content being viewed by others
References
Mariette C, Piessen G, Briez N, Gronnier C, Triboulet JP (2011) Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol 12:296–305. https://doi.org/10.1016/S1470-2045(10)70125-X
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D (2016) Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:50–57. https://doi.org/10.1093/annonc/mdw329
Lledo G, Mariette C, Raoul JL, Dahan L, Landi B, Conroy T, et al. (2016) Cancer de l’oesophage. https://www.snfge.org/content/1-cancer-de-loesophage. Accessed 25 July 2022
Kehlet H, Wilmore DW (2002) Multimodal strategies to improve surgical outcome. Am J Surg 183:630–641. https://doi.org/10.1016/S0002-9610(02)00866-8
Wilmore DW, Kehlet H (2001) Management of patients in fast track surgery. BMJ 322:473–476. https://doi.org/10.1136/bmj.322.7284.473
Kehlet H, Wilmore DW (2008) Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 248:189–198. https://doi.org/10.1097/SLA.0b013e31817f2c1a
Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ (2011) Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. CD007635. https://doi.org/10.1002/14651858.CD007635.pub2
Wang D, Kong Y, Zhong B, Zhou X, Zhou Y (2010) Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg 14:620–627. https://doi.org/10.1007/s11605-009-1139-5
Findlay JM, Gillies RS, Millo J, Sgromo B, Marshall REK, Maynard ND (2014) Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 259:413–431. https://doi.org/10.1097/SLA.0000000000000349
Dorcaratto D, Grande L, Pera M (2013) Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg 30:70–78. https://doi.org/10.1159/000350701
Andreou A, Biebl M, Dadras M, Struecker B, Sauer IM, Thuss-Patience PC et al (2016) Anastomotic leak predicts diminished long-term survival after resection for gastric and esophageal cancer. Surgery 160:191–203. https://doi.org/10.1016/j.surg.2016.02.020
Lagarde SM, de Boer JD, ten Kate FJW, Busch ORC, Obertop H, van Lanscht JJB (2008) Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg 247:71–76. https://doi.org/10.1097/SLA.0b013e31815b695e
Markar S, Gronnier C, Duhamel A, Mabrut JY, Bail JP, Carrere N et al (2015) The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg 262:972–980. https://doi.org/10.1097/SLA.0000000000001011
Warschkow R, Beutner U, Steffen T, Müller SA, Schmied BM, Güller U et al (2012) Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg 256:245–250. https://doi.org/10.1097/SLA.0b013e31825b60f0
Lagoutte N, Facy O, Ravoire A, Chalumeau C, Jonval L, Rat P et al (2012) C-reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg 149:345–349. https://doi.org/10.1016/j.jviscsurg.2012.09.003
Facy O, Paquette B, Orry D, Binquet C, Masson D, Bouvier A et al (2016) Diagnostic accuracy of inflammatory markers as early predictors of infection after elective colorectal surgery: results from the IMACORS study. Ann Surg 263:961–966. https://doi.org/10.1097/SLA.0000000000001303
Ortega-Deballon P, Radais F, Facy O, d’Athis P, Masson D, Charles PE et al (2010) C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg 34:808–814. https://doi.org/10.1007/s00268-009-0367-x
Singh PP, Zeng ISL, Srinivasa S, Lemanu DP, Connolly AB, Hill AG (2014) Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 101:339–346. https://doi.org/10.1002/bjs.9354
Gordon AC, Cross AJ, Foo EW, Roberts RH (2018) C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg 88:223–227. https://doi.org/10.1111/ans.13681
Van Genderen ME, Lima A, de Geus H, Klijn E, Wijnhoven B, Gommers D et al (2011) Serum C-reactive protein as a predictor of morbidity and mortality in intensive care unit patients after esophagectomy. Ann Thorac Surg 91:1775–1779. https://doi.org/10.1016/j.athoracsur.2011.02.042
Hoeboer SH, Groeneveld ABJ, Engels N, van Genderen M, Wijnhoven BPL, van Bommel (2015) Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg 19:613–624. https://doi.org/10.1007/s11605-015-2745-z
Dutta S, Fullarton GM, Forshaw MJ, Horgan PG, McMillan DC (2011) Persistent elevation of C-reactive protein following esophagogastric cancer resection as a predictor of postoperative surgical site infectious complications. World J Surg 35:1017–1025. https://doi.org/10.1007/s11605-015-2745-z
Miki Y, Toyokawa T, Kubo N, Tamura T, Sakurai K, Tanaka H et al (2017) C-reactive protein indicates early stage of postoperative infectious complications in patients following minimally invasive esophagectomy. World J Surg 41:796–803. https://doi.org/10.1007/s00268-016-3803-8
Warschkow R, Tarantino I, Ukegjini K, Beutner U, Müller SA, Schmied BM et al (2012) Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg 397:727–736. https://doi.org/10.1007/s00423-012-0944-6
Asti E, Bonitta G, Melloni M, Tornese S, Milito P, Sironi A et al (2018) Utility of C-reactive protein as predictive biomarker of anastomotic leak after minimally invasive esophagectomy. Langenbecks Arch Surg 403:235–244. https://doi.org/10.1007/s00423-018-1663-4
Li H, Wang D, Wei W, Ouyang L, Lou N (2019) The predictive value of coefficient of PCT × BG for anastomotic leak in esophageal carcinoma patients with ARDS after esophagectomy. J Intensive Care Med 34:572–527. https://doi.org/10.1177/0885066617705108
Aiolfi A, Asti E, Rausa E, Bonavina G, Bonitta G, Bonavina L (2018) Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: systematic review and Bayesian meta-analysis. PLoS ONE 13:e0209272. https://doi.org/10.1371/journal.pone.0209272
Siewert JR, Feith M, Stein HJ (2005) Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol 90:139–146. https://doi.org/10.1002/jso.20218
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol 17:1471–1474. https://doi.org/10.1245/s10434-010-0985-4
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 262:286–294. https://doi.org/10.1097/SLA.0000000000001098
Calandra T, Cohen J (2005) The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 33(1538–1548):1. https://doi.org/10.1097/01.ccm.0000168253.91200.83
Briez N, Piessen G, Bonnetain F, Brigand C, Carrere N, Collet D et al (2011) Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer 11:310. https://doi.org/10.1186/1471-2407-11-310
Romain B, Chemaly R, Meyer N, Chilintseva N, Triki E, Brigand C et al (2014) Diagnostic markers of postoperative morbidity after laparoscopic Roux-en-Y gastric bypass for obesity. Langenbecks Arch Surg 399:503–508. https://doi.org/10.1007/s00423-014-1180-z
Messager M, Warlaumont M, Renaud F, Marin H, Branche J, Piessen G et al (2017) Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 43:258–269. https://doi.org/10.1016/j.ejso.2016.06.394
Liou DZ, Serna-Gallegos D, Mirocha J, Bairaman V, Alban RF, Soukiasian HJ (2018) Predictors of failure to rescue after esophagectomy. Ann Thorac Surg 105:871–878. https://doi.org/10.1016/j.athoracsur.2017.10.022
Okholm C, Goetze JP, Svendsen LB, Achiam MP (2014) Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol 49:1027–1034. https://doi.org/10.3109/00365521.2014.917698
Babic B, Tagkalos E, Gockel I, Corvinus F, Hadzijusufovic E, Hoppe-Lotichius M et al (2020) C-reactive protein levels after esophagectomy are associated with increased surgical trauma and complications. Ann Thorac Surg 109:1574–1583. https://doi.org/10.1016/j.athoracsur.2019.12.016
Facy O, Paquette B, Orry D, Santucci N, Rat P, Rat P et al (2017) Inflammatory markers as early predictors of infection after colorectal surgery: the same cut-off values in laparoscopy and laparotomy? Int J Colorectal Dis 32:857–863. https://doi.org/10.1007/s00384-017-2805-9
Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N (2001) Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg 88:1346–1351. https://doi.org/10.1046/j.0007-1323.2001.01918.x
Park JK, Kim JJ, Moon SW (2017) C-reactive protein for the early prediction of anastomotic leak after esophagectomy in both neoadjuvant and non-neoadjuvant therapy case: a propensity score matching analysis. J Thorac Dis 9:3693–3702. https://doi.org/10.21037/jtd.2017.08.125
Durila M, Bronský J, Haruštiak T, Pazdro A, Pechová M, Cvachovec K (2012) Early diagnostic markers of sepsis after oesophagectomy (including thromboelastography). BMC Anesthesiol 12:12. https://doi.org/10.1186/1471-2253-12-12
Ito S, Sato N, Kojika M, Yaegashi Y, Suzuki Y, Suzuki K et al (2005) Serum procalcitonin levels are elevated in esophageal cancer patients with postoperative infectious complications. Eur Surg 37:22–28. https://doi.org/10.1159/000083144
Tang BMP, Eslick GD, Craig JC, McLean AS (2007) Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 7:210–217. https://doi.org/10.1016/S1473-3099(07)70052-X
Cousin F, Ortega-Deballon P, Bourredjem A, Doussot A, Giaccaglia V, Fournel I (2016) Diagnostic accuracy of procalcitonin and C-reactive protein for the early diagnosis of intra-abdominal infection after elective colorectal surgery: a meta-analysis. Ann Surg 264:252–256. https://doi.org/10.1097/SLA.0000000000001545
Noble F, Curtis N, Harris S, Kelly JJ, Bailey IS, Byrne JP et al (2012) Risk assessment using a novel score to predict anastomotic leak and major complications after oesophageal resection. J Gastrointest Surg 16:1083–1095. https://doi.org/10.1007/s11605-012-1867-9
De Magistris L, Paquette B, Orry D, Facy O, Di Giacomo G, Rat P et al (2016) Preoperative inflammation increases the risk of infection after elective colorectal surgery: results from a prospective cohort. Int J Colorectal Dis 31:1611–1617. https://doi.org/10.1007/s00384-016-2620-8
Acknowledgements
This paper is dedicated to the memory of Professor Christophe Mariette (surgeon from Lille) who was involved at the very beginning of this study. The authors thank Professor Christine Binquet for his help in statistics, Professor Pascal Hammel for his advice, and Professor Abe Fingerhut for English-editing.
Funding
This work was supported by grants from the Agence Nationale de la Recherche (LabEx LipSTIC, ANR-11-LABX-0021) as well as from the Bourgogne-Franche-Comté Region, the Fond Européen de Développement Regional (FEDER), and the Dijon-Bourgogne University Hospital.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Paul Rat, Marguerite Vanderbeken, and Alexandre Chebaro. Analysis and interpretation of data were performed by Paul Rat, Guillaume Piessen, Olivier Facy, Patrick Rat, Cyril Boisson, and Pablo Ortega-Deballon. The first draft of the manuscript was written by Paul Rat and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rat, P., Piessen, G., Vanderbeken, M. et al. C-reactive protein identifies patients at low risk of anastomotic leak after esophagectomy. Langenbecks Arch Surg 407, 3377–3386 (2022). https://doi.org/10.1007/s00423-022-02703-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02703-5