Abstract
Purpose
Since their invention 40 years ago, totally implantable venous-access ports (TIVAPs) have become indispensable in cancer treatment. The aim of our study was to analyze complications under standardized operative and perioperative procedures and to identify risk factors for premature port catheter explantation.
Methods
A total of 1008 consecutive TIVAP implantations were studied for success rate, perioperative, early, and late complications. Surgical, clinical, and demographic factors were analyzed as potential risk factors for emergency port catheter explantation.
Results
Successful surgical TIVAP implantation was achieved in 1005/1008 (99.7%) cases. No intraoperative or perioperative complications occurred. A total of 32 early complications and 88 late complications were observed leading to explantation in 11/32 (34.4%) and 34/88 (38.6%) cases, respectively. The most common complications were infections in 4.7% followed by thrombosis in 3.6%. Parameters that correlated with unplanned TIVAP explantation were gender (port in situ: female 95% vs. male 91%, p = 0.01), underlying disease (breast cancer 97% vs. gastrointestinal 89%, p = 0.004), indication (chemotherapy 95% vs. combination of chemotherapy and parenteral nutrition 64%, p < 0.0001), and type of complication (infection 13.4% vs. TIVAP-related complication 54% and thrombosis 95%, p < 0.0001).
Conclusion
Standardized operative and perioperative TIVAP implantation procedures provide excellent results and low explantation rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forty years ago, the first totally implantable venous-access ports (TIVAPs) were developed by Niederhuber and meanwhile TIVAPs are essential in cancer treatment [1]. The rate of implanted TIVAPs is still constantly rising because of the increasing incidence of oncological malignancies and the development of new multimodal therapy regimens. These ports permit safe long-term administration of chemotherapeutic agents, parenteral nutrition, and antimicrobial treatment [2]. Because of these versatile applications, they are suitable not only in solid-tumor cancers and hematological malignancies, but also in chronic disease such as cystic fibrosis and HIV [3, 4].
Despite the fact that TIVAPs offer many advantages like reliability and safety, especially for cancer patients, TIVAP-associated complications may occur and require early diagnosis and treatment. Since their invention four decades ago, complications have been identified and analyzed and practical guidelines created [4, 5, 6].
Initially, implantation technique and surgical complications were mostly in focus. Comparison of the two alternative approaches showed the success rate reported in different studies to be clearly in favor of the Seldinger technique, namely between 90 and 100% [7, 8, 9, 10, 11, 12], while the success rate for venous cutdown was only 70–94% [13, 14, 15, 16].
With further development of equipment and improvement of surgical technique, the most frequent complications changed to catheter-associated infections and thrombosis [17, 18]. The current literature reports complication rates between 6.9 and 17.7% [19, 20]. In the worst case, TIVAP-associated complications lead to TIVAP explantation. Fortunately, the TIVAP explantation rate described in recent studies is low with a few exceptions [21, 22]. Nevertheless, every single explantation means notable consequences for every patient, particularly a delay in ongoing chemotherapy for cancer treatment and difficulties for parenteral nutrition, resulting in increased morbidity, mortality, and costs [23, 24].
The aim of our study was to analyze complications under standardized operative and perioperative procedures and to identify risk factors for premature port catheter explantation.
Material and methods
The retrospective cohort study was approved by Tuebingen University Ethics Committee (192/2018B02). The study cohort consisted of 1008 consecutive TIVAP implantations performed by one surgeon (G. M.) in patients aged 16 or older who received a TIVAP between January 1, 2016 and October 31, 2017 at the ambulatory operative center of the Department of General, Visceral, and Transplant Surgery, Tuebingen University Hospital, Germany. We retrospectively screened all patients treated with operation and procedure score OPS 5–399.5 (port implantation). Follow-up continued until the TIVAP was removed or the patient died. Follow-up time ended on October 31, 2018, so that patients were followed for minimum 1 year following implantation.
Surgical procedure and standard anti-thrombosis prophylaxis
All operations were performed by the same high-volume general surgeon (G. M.) in local anesthesia using the well-established standardized open technique, i.e., cephalic vein cutdown [25].
Postoperatively patients were observed in the recovery room. After a chest X-ray was performed to confirm correct positioning of the TIVAP and to exclude pneumothorax, the patients were discharged.
A standard anti-thrombosis prophylaxis of low molecular weight heparin s.c., Fragmin P® 2.500 IU per day during the first 3 weeks after implantation, was recommended for all patients treated at our center. After every use and at least every 12 weeks, the system was flushed and blocked with 10–20 ml of NaCl 0.9%.
No perioperative antibiotic prophylaxis was given and immediate use of the TIVAP was allowed.
Definitions of complications
Complications were defined as perioperative (during implantation and within 24 h after implantation), early (> 24 h and < 30 days after implantation), or delayed (more than 30 days after implantation). Classification of infections was performed according to the published guidelines [22]. Local infection was defined as redness, swelling, pain, and tenderness without signs of systemic infection. In cases involving fever and positive paired blood cultures from the central line and the peripheral vein with the same micro-organism, systemic inflammatory response syndrome/sepsis was diagnosed. Thrombosis was defined either clinically or radiologically (vascular sonogram or computed tomography (CT)). Thrombosis was differentiated as port chamber thrombosis, port tip thrombosis, or deep branch vein thrombosis. TIVAP-related complications consist of dysfunction, dislocation, disconnection, and fracture. Patient-related complications comprise hematoma, seroma, skin perforation, erythema, and pain without clinical signs of infection.
Data collection and statistics
Data were collected with the hospital information database (SAP for Healthcare; SAP AG Walldorf). Collected data for each patient comprise demographic characteristics including age, gender, underlying disease, indication for permanent central venous catheter placement (chemotherapy, parenteral nutrition, combination), and follow-up time. In addition, the following surgical data were obtained: date of implantation, implantation side (right/left), operation time, implantation technique, and selected vein. Follow-up data consisted of complications (date, category, and treatment), indication, and date of TIVAP explantation. In the case of infection, pathogens were registered.
Mean values were compared with the Wilcoxon test (JMP® 14.0, SAS Institute, Cary, NC, USA). Complication rates were reported in absolute numbers, as rate per 1000 catheter days and percentage. Catheter survival was defined as the presence of the originally implanted catheter. Event was “TIVAP explantation due to complications”. Death with functioning TIVAP and planned explantation due to termination of therapy were considered censoring events. TIVAP survival curves were calculated with the Kaplan–Meier method and tested for significance using a log-rank test. A p value < 0.05 was considered significant. Results reported in the article, tables, and figures are reported as mean ± standard error of mean (SEM).
Results
Altogether 1021 TIVAP procedures were screened (Fig. 1); 13 procedures had to be excluded because they did not meet the inclusion criteria. In nine cases, the OPS score 5–399.5 was erroneously used for TIVAP revision and in one case for TIVAP explantation. Three operations were performed by a different surgeon.
Successful surgical TIVAP implantation was achieved in 1005/1008 (99.7%) cases in 991 patients (14 patients received a second catheter). The following presented data refer to these 1005 successful implantations. Demographic and clinical characteristics are shown in Table 1. Mean age was 59 ± 0.5 (range 16–91) years, 742 (73.8%) patients were female, and 263 (26.2%) were male. The most prevalent underlying diseases requiring TIVAP implantation were breast carcinoma (432/1005, 43%), gastrointestinal carcinoma (252/1005, 25%), and gynecological tumors (142/1005, 14.1%).
Most patients received a TIVAP for the administration of chemotherapy (961/1005, 95.6%), and 21 (2.1%) patients needed chemotherapy combined with parenteral nutrition.
Implantation side was mainly right (607 operations, 60.4%). The preferred blood vessel was the Vena cephalica in 958 (95.3%) cases. The Vena jugularis externa was used in 46 (4.6%) patients. In 10% of the implantations (also in the vein cutdown technique), a guidewire was additionally used to position the catheter tube.
Mean operation time was 30 ± 0.4 min (range 15–136 min). No intraoperative or perioperative complications occurred.
Overall, 120 (12%) complications were observed during the follow-up time of altogether 611.691 catheter days. Complications are summarized in Table 2. They comprised 32 (26.7%) early and 88 (73.3%) late complications. The explantation rate due to complications was similar for early (11/32, 34.4%) and late (34/88, 38.6%) complications. The most common complications were infections, which occurred in 47/1005 (4.7%) of the TIVAPs. Port catheter-induced blood stream infections were observed in 32 (3.2%; 0.052/1000 catheter days) cases and mainly occurred as a late complication 27/32 (84.4%). On average, blood stream infection happened after 179 ± 32 days. Conservative antibiotic therapy was successful in 11/32 (34.4%) cases, and 21/32 (65.6%) catheters had to be explanted due to systemic infection. Port pocket infections were reported in 15/1005 (1.5%; 0.024/1000 catheter days) TIVAPs, 5/15 (33%) were early complications, and 10/15 (67%) were late complications. On average, port pocket infection occurred after 166 ± 57 days. Therapy consisted of 12 (80%) explantations and antibiotic therapy in three cases (20%). With regard to disease, infections were mostly seen in patients with leukemia/lymphoma (5 infections/47 patients; 10.6%), followed by gastrointestinal cancer (22/252; 8.7%) and breast cancer (10/432; 2.3%). For a more detailed analysis of infections in the group of gastrointestinal cancer patients, a breakdown of diseases was performed: Infections were reported most frequently in gastric cancer (5/21; 23.8%), followed by pancreas carcinoma (9/56; 16.1%) and rectum carcinoma (1/33; 0.3%). Of the patients with rectum carcinoma, 17/33 (51.5%) had a diverting stoma. None of these patients suffered from an infection. Both local and systemic infections were mainly caused by Staphylococcus epidermidis (15; 32%), Staphylococcus aureus (12; 26%), and Escherichia coli (5; 11%). Temporal occurrence of pathogens shows differences: Staphylococcus aureus was mainly responsible for early infections, on average after 76 ± 33 days. Staphylococcus epidermidis and E. coli were found later after 264 ± 83 and after 382 ± 99 days (Wilcoxon test p = 0.0109 and p = 0.0302), respectively.
The second most common complication was thrombosis, which was evident in 36/1005 (3.6%) cases, corresponding to 0.06/1000 catheter days. Of the thromboses, 5/36 (13.9%) were early complications and 2/36 occurred during the first 3-week phase of recommended anticoagulation, and 31/36 (86.1%) were late complications. Average time of occurrence was 192 ± 35 days. Localizations of the thrombus were the port chamber in three (8.3%) cases, port tip in five (13.9%), and deep branch vein in 28 (77.8%) cases. Nearly half of the patients (17/36; 47.2%) were asymptomatic and thrombosis was diagnosed as an incidental finding in staging computed tomography. In two patients (5.6%), thrombosis resulted in segmental lung embolism. In 35/36 (92.2%) thromboses, conservative anticoagulative therapy was successful. In one of the two patients with a pulmonary embolism, the TIVAP had to be removed because the patient was already anticoagulated when the lung embolism occurred.
In view of underlying disease, thrombosis was found mainly in the group of leukemia/lymphoma patients, namely in 2/47 (4.3%), followed by breast carcinoma 18/435 (4.1%) and gastrointestinal tumor 9/252 (3.6%) patients.
TIVAP-related complications were documented in 20/1005 (2%) cases, corresponding to 0.033/1000 catheter days; 7/20 (35%) were classified as early and 13/20 (65%) as late complications. Most frequently dislocation of the catheter was seen in 8/20 (40%), followed by fracture in 5/20 (25%) cases and dysfunction also in five cases. Therapy consisted of explantation (7/20, 35%), revision (7/20, 35%), and conservative therapy (6/20, 30%).
Patient-related complications occurred in 17/1005 (1.7%) patients, corresponding to 0.028/1000 catheter days. The majority of the complications occurred early (10/17, 58.8%), namely hematoma and seroma. No pneumothoraces were detected. Skin perforation was seen clearly later (131–337 days after implantation) in four patients and resulted in explantation in all cases. Of the patient-related complications, 12/17 were treated conservatively with local therapy and analgesia.
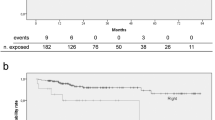
At the end of the observation period, 805/1005 (80%) of the implanted TIVAPs were functioning in situ (Fig. 2).
During the study period, 63 patients died with a functioning TIVAP. Of 137 TIVAP explantations, 82 (59.9%) were according to plan after completion of the therapy regimen, while 55 (40.1%) TIVAPs had to be removed due to complications. Explantation due to complications was indicated after approximately 179 ± 25 days and therefore TIVAP in situ time was significantly shorter than for planned explantations after 379 ± 20 days (p < 0.0001).
Kaplan–Meier curves for TIVAP survival in relation to the event “explantation due to complication” are shown in Fig. 3. Parameters that correlated with TIVAP explantation were gender (Fig. 3a) (port in situ: female 95% vs. male 91%, p = 0.01), underlying disease (Fig. 3b) (breast carcinoma 97% vs. gastrointestinal 89%, p = 0.004), indication (Fig. 3c) (chemotherapy 95% vs. combination of chemotherapy and parenteral nutrition 64%, p < 0.0001), and type of complication (Fig. 3d) (infection 13.4% vs. TIVAP-related complication 54% and thrombosis 95%, p < 0.0001). In contrast, catheter survival was not affected by implantation side, operation time less or more than 30 min, with or without the need for a guidewire, or by patient age.
Kaplan–Meier curves for catheter survival. a) relevant factor: gender; female: dotted line, male: black line, * p = 0.01. b) relevant factor: disease; breast cancer: black line, gastrointestinal cancer: grey line, leukemia/lymphoma: dotted line, * p = 0.004 breast cancer vs. gastrointestinal cancer. c) relevant factor: indication; chemotherapy: dotted black line, parenteral nutrition: grey line, combination of chemotherapy and parenteral nutrition: dotted and dashed grey line, * p < 0.001 chemotherapy vs. chemotherapy and parenteral nutrition. d) relevant factor: type of complication; thrombosis: black line, TIVAP-related complication: grey line, infection: dotted black line, * p < 0.0001 thrombosis vs. TIVAP-related complication, * p < 0.0001 thrombosis vs. infection
Discussion
In the present study, more than 1000 TIVAP implantations were analyzed in order to identify risk factors for premature catheter explantation. The surgical success rate of 99.7% in our series and the large cohort provide an excellent foundation for further analysis. The extremely low failure rate, the absence of intraoperative and perioperative complications, and the consistently short operating time of 30 ± 0.4 min in our study might be caused by the fact that all operations were performed by the same highly experienced surgeon.
The overall complication rate in our study was low at 0.196 complications per 1000 days or 12% altogether. In accordance with other studies, the temporal distribution was in favor of late complications (8.8%) as compared to early complications (3.2%) [17, 19]. From this, it follows that only 5.5% of the implanted TIVAPs had to be explanted due to complications. The range in other series varies between 4.3 and 29.8% [20, 21, 22, 26]. The high explantation rate of 29.8% in the study by Kilic et al. might be explained by their high infection rate of 16.1% [26]. In our study, infections occurred in 4.7% of TIVAPs and are the most common complication as well as the most common reason for TIVAP explantation. It must be underscored that TIVAPs had to be explanted in only two-thirds of patients with a systemic infection as compared to clearly higher explantation rates reported by Vida et al. (81%), Ahn et al. (88%), and Teichgräber et al. (100%) [17, 27, 28]. The importance of early diagnosis and therapy of infections was proven by Mermel et al. [29]. For TIVAP implantation in our hospital, no perioperative antibiotic is administered and the applicability of this procedure was confirmed by our study data with overall only five local and five systemic early infections. Infection rates in recent studies vary between 1.6 and 50% [17, 19, 20, 30, 31]. The lowest infection rate was found by Ma et al. in a study cohort consisting of only breast cancer patients [19], and this aspect was confirmed in the present data showing a low infection rate of 2.3% for this patient group. The highest infection rate was found by Viana Taveira in pediatric patients, and nearly 70% of whom had lymphoma/leukemia [31]. In accordance with these data, the small group of patients with lymphoma/leukemia in our study showed the highest infection rate, namely 10.6%. Increased infection rates in hemato-oncology malignancies were reported in several studies and intensive chemotherapy and impairment of the immune system were seen as a causal relationship [28, 30, 32]. Zerati et al. and Shim et al. justify their increased infection rate with the high rate (20.5%) of stationary patients [22, 30]. In our study, all operations were performed on an out-patient basis, which might be beneficial for a lower infection rate.
In the present study, the infection rate for patients with gastrointestinal cancer was 8.7% and was reported most frequently in patients with gastric cancer (23.8%), followed by pancreas carcinoma (16.1%) and rectal carcinoma (0.3%). Half of the patients with rectal carcinoma in our study had a diverting stoma, but none of these patients came down with an infection.
Of the infections, 79% were caused by gram-positive pathogens, mainly Staphylococcus epidermidis and Staphylococcus aureus, which is consistent with the findings of other current studies [27, 31, 33]. A shift toward gram-positive bacteremia in cancer patients was observed many decades ago and not only the use of antibiotic prophylaxis but also the existence of an indwelling catheter and the nature of chemotherapy are held responsible for this [34, 35].
The second most common complication observed in our study was thrombosis in 3.6% of TIVAPs. Standard anti-thrombosis prophylactic regimen in our center consists of the recommendation to administer low molecular weight heparin during the first 3 weeks after implantation and to flush and block the system with 10–20 ml of NaCl 0.9% after every use and at least every 12 weeks. Two thromboses occurred during the first 3-week phase of recommended anticoagulation. Since this is a retrospective study, it is unknown if the patient followed the recommendation. The primary prevention of TIVAP-associated thrombosis with anticoagulant drugs is currently not recommended by most scientific societies and guidelines [36, 37]. Instead, risk stratification for previous thrombosis, type, location, and stage of cancer and hereditary defects is suggested [38]. It is debatable whether the recommendation for anti-thrombosis prophylaxis at our center is too general and some patients received it unnecessarily. The results of our study show that the standard anti-thrombosis prophylactic regimen at our center needs to be discussed because it appears to provide little benefit and therefore could be changed to a risk-stratified regimen.
Nearly half (47.2%) of the patients were asymptomatic and thrombosis was diagnosed as an incidental finding in staging computed tomography. Asymptomatic thrombosis was also recorded in prospective studies and incidences of 71% and 94% were reported (21, 68). In only one case of thrombosis resulting in a segmental lung embolism during anticoagulative therapy did the TIVAP have to be removed. All other thromboses were treated successfully with anticoagulative therapy.
In three cases, thrombi were localized in the port chamber, which in the proper meaning of the word is not really a thrombosis, but more probably a consequence of poor nursing practices with insufficient push-pause cleaning of the line after blood sampling.
Although TIVAP-related complications occurred very rarely, namely in only 2% of TIVAPs, their occurrence significantly affected catheter survival. In contrast, all patient-related complications were able to be treated conservatively with the exception of skin perforation in four cases when a TIVAP had to be explanted.
Conclusion
In conclusion, our large single-center series shows that standardized operative and perioperative procedures for TIVAP implantation provide excellent results and a low explantation rate. Risk factors for unplanned explantation were gender, underlying disease, indication, and kind of complication.
References
Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E (1982) Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 92:706–712
Lebeaux D, Fernandez-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B, Beloin C (2014) Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 14:146–159
Vescia S, Baumgartner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, Harbeck N (2008) Management of venous port systems in oncology: a review of current evidence. Ann Oncol 19:9–15
Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M, Espen, (2009) ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr 28:365–377
Hentrich M, Schalk E, Schmidt-Hieber M, Chaberny I, Mousset S, Buchheidt D, Ruhnke M, Penack O, Salwender H, Wolf HH, Christopeit M, Neumann S, Maschmeyer G, Karthaus M, Infectious Diseases Working Party of the German Society of H, Medical O (2014) Central venous catheter-related infections in hematology and oncology: 2012 updated guidelines on diagnosis, management and prevention by the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology. Ann Oncol 25:936–947
Sousa B, Furlanetto J, Hutka M, Gouveia P, Wuerstlein R, Mariz JM, Pinto D, Cardoso F, Committee EG (2015) Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann Oncol 26(Suppl 5):v152-168
Nocito A, Wildi S, Rufibach K, Clavien PA, Weber M (2009) Randomized clinical trial comparing venous cutdown with the Seldinger technique for placement of implantable venous access ports. Br J Surg 96:1129–1134
Morris SL, Jaques PF, Mauro MA (1992) Radiology-assisted placement of implantable subcutaneous infusion ports for long-term venous access. Radiology 184:149–151
Shetty PC, Mody MK, Kastan DJ, Sharma RP, Burke MW, Venugopal C, Burke TH (1997) Outcome of 350 implanted chest ports placed by interventional radiologists. J Vasc Interv Radiol 8:991–995
Kluge A, Stroh H, Wagner D, Rauber K (1998) The fluoroscopy-guided implantation of subcutaneous venous ports: the complications and long-term results. Rofo 169:63–67
Lorch H, Zwaan M, Kagel C, Weiss HD (2001) Central venous access ports placed by interventional radiologists: experience with 125 consecutive patients. Cardiovasc Intervent Radiol 24:180–184
Simpson KR, Hovsepian DM, Picus D (1997) Interventional radiologic placement of chest wall ports: results and complications in 161 consecutive placements. J Vasc Interv Radiol 8:189–195
Torramade JR, Cienfuegos JA, Hernandez JL, Pardo F, Benito C, Gonzalez J, Balen E, de Villa V (1993) The complications of central venous access systems: a study of 218 patients. Eur J Surg 159:323–327
Di Carlo I, Cordio S, La Greca G, Privitera G, Russello D, Puleo S, Latteri F (2001) Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 136:1050–1053
Davis SJ, Thompson JS, Edney JA (1984) Insertion of Hickman catheters. A comparison of cutdown and percutaneous techniques. Am Surg 50:673–676
Au FC (1989) The anatomy of the cephalic vein. Am Surg 55:638–639
Teichgraber UK, Kausche S, Nagel SN, Gebauer B (2011) Outcome analysis in 3,160 implantations of radiologically guided placements of totally implantable central venous port systems. Eur Radiol 21:1224–1232
Voog E, Campion L, du Rusquec P, Bourgeois H, Domont J, Denis F, Emmanuel E, Dupuis O, Ganem G, Lafont C, Le Du K, Pavluc E, Pointreau Y, Roche S, Juhel-Voog L, Zinger M, Solal-Celigny P (2018) Totally implantable venous access ports: a prospective long-term study of early and late complications in adult patients with cancer. Support Care Cancer 26:81–89
Ma LI, Liu Y, Wang J, Chang Y, Yu L, Geng C (2016) Totally implantable venous access port systems and associated complications: a single-institution retrospective analysis of 2,996 breast cancer patients. Mol Clin Oncol 4:456–460
Wolosker N, Yazbek G, Nishinari K, Malavolta LC, Munia MA, Langer M, Zerati AE (2004) Totally implantable venous catheters for chemotherapy: experience in 500 patients. Sao Paulo Med J 122:147–151
Fischer L, Knebel P, Schroder S, Bruckner T, Diener MK, Hennes R, Buhl K, Schmied B, Seiler CM (2008) Reasons for explantation of totally implantable access ports: a multivariate analysis of 385 consecutive patients. Ann Surg Oncol 15:1124–1129
Shim J, Seo TS, Song MG, Cha IH, Kim JS, Choi CW, Seo JH, Oh SC (2014) Incidence and risk factors of infectious complications related to implantable venous-access ports. Korean J Radiol 15:494–500
Pinelli F, Cecero E, Degl’Innocenti D, Selmi V, Giua R, Villa G, Chelazzi C, Romagnoli S, Pittiruti M (2018) Infection of totally implantable venous access devices: a review of the literature. J Vasc Access 19:230–242
Biffi R, Pozzi S, Bonomo G, Della Vigna P, Monfardini L, Radice D, Rotmensz N, Zampino MG, Fazio N, Orsi F (2014) Cost effectiveness of different central venous approaches for port placement and use in adult oncology patients: evidence from a randomized three-arm trial. Ann Surg Oncol 21:3725–3731
Povoski SP (2000) A prospective analysis of the cephalic vein cutdown approach for chronic indwelling central venous access in 100 consecutive cancer patients. Ann Surg Oncol 7:496–502
Kilic S, Soyer T, Karnak I, Ciftci AO, Tanyel FC, Senocak ME (2016) Evaluation of the removal reasons of totally implantable venous devices in children: a retrospective study. Turk J Pediatr 58:187–194
Vidal M, Genillon JP, Forestier E, Trouiller S, Pereira B, Mrozek N, Aumeran C, Lesens O (2016) Outcome of totally implantable venous-access port-related infections. Med Mal Infect 46:32–38
Ahn SJ, Kim HC, Chung JW, An SB, Yin YH, Jae HJ, Park JH (2012) Ultrasound and fluoroscopy-guided placement of central venous ports via internal jugular vein: retrospective analysis of 1254 port implantations at a single center. Korean J Radiol 13:314–323
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45
Zerati AE, Figueredo TR, de Moraes RD, da Cruz AM, da Motta-Leal Filho JM, Freire MP, Wolosker N, de Luccia N (2016) Risk factors for infectious and noninfectious complications of totally implantable venous catheters in cancer patients. J Vasc Surg Venous Lymphat Disord 4:200–205
Viana Taveira MR, Lima LS, de Araujo CC, de Mello MJ (2017) Risk factors for central line-associated bloodstream infection in pediatric oncology patients with a totally implantable venous access port: a cohort study. Pediatr Blood Cancer 64:336–342
Samaras P, Dold S, Braun J, Kestenholz P, Breitenstein S, Imhof A, Renner C, Stenner-Liewen F, Pestalozzi BC (2008) Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology 74:237–244
Okada S, Shiraishi A, Yamashiro Y, Inoue T, Tsuge D, Aida M, Kuwatsuru R (2015) A retrospective statistical analysis of the late complications associated with central venous port placements. Jpn J Radiol 33:21–25
Zinner SH (1999) Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis 29:490–494
Ramphal R (2004) Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis 39(Suppl 1):S25-31
Debourdeau P, KassabChahmi D, Le Gal G, Kriegel I, Desruennes E, Douard MC, Elalamy I, Meyer G, Mismetti P, Pavic M, Scrobohaci ML, Levesque H, Renaudin JM, Farge D, working group of the SOR (2009) 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol 20:1459–1471
Kahale LA, Tsolakian IG, Hakoum MB, Matar CF, Barba M, Yosuico VE, Terrenato I, Sperati F, Schunemann H, Akl EA (2018) Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst Rev 6:CD006468
Debourdeau P, Farge D, Beckers M, Baglin C, Bauersachs RM, Brenner B, Brilhante D, Falanga A, Gerotzafias GT, Haim N, Kakkar AK, Khorana AA, Lecumberri R, Mandala M, Marty M, Monreal M, Mousa SA, Noble S, Pabinger I, Prandoni P, Prins MH, Qari MH, Streiff MB, Syrigos K, Buller HR, Bounameaux H (2013) International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost 11:71–80
Acknowledgements
The authors thank Mary Heaney Margreiter for her kind contribution to the preparation of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Sarah Kalmbach, Martin Schenk, Christian Thiel, and Karolin Thiel. The operator was Gerhard Maier. The first draft of the manuscript was written by Christian Thiel and Karolin Thiel. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thiel, K., Kalmbach, S., Maier, G. et al. Standardized procedure prevents perioperative and early complications in totally implantable venous-access ports—a complication analysis of more than 1000 TIVAP implantations. Langenbecks Arch Surg 407, 3755–3762 (2022). https://doi.org/10.1007/s00423-022-02656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02656-9