Abstract
Purpose
The tobacco epidemic is one of the biggest global public health issues impacting quality of life and surgical outcomes. Although 30% of colon cancers warrant a right hemicolectomy (RH), there is no specific data on the influence of smoking on postoperative complications following RH for cancer. The aim of this study was to determine its effect on post-surgical outcomes.
Methods
Patients who underwent elective RH for colon cancer between 2016 and 2019 were identified from the ACS-NSQIP database. Propensity score matching (PSM) was used with a maximum absolute difference of 0.05 between propensity scores. Primary outcome was to assess the 30-day complication risk profile between smokers and non-smokers. Secondary outcomes included smoking impact on wound and major medico-surgical complication rates, as well as risk of anastomotic leak (AL) using multivariable logistic regression models.
Results
Following PSM, 5652 patients underwent RH for colon cancer with 1,884 (33.3%) identified as smokers. Smokers demonstrated a higher rate of organ space infection (4.1% vs 3.1%, p = 0.034), unplanned return to theatre (4.8% vs 3.7%, p = 0.045) and risk of AL (3.5% vs 2.1%, p = 0.005). Smoking was found to be an independent risk factor for wound complications (OR 1.32, 95% CI 1.03–1.71, p = 0.032), primary pulmonary complications (OR 1.50, 95% CI 1.06–2.13, p = 0.024) and AL (OR 1.66, 95% CI 1.19–2.31, p = 0.003).
Conclusion
Smokers have increased risk of developing major post-operative complications compared to non-smokers. Clinicians and surgeons must inform smokers of these surgical risks and potential benefit of smoking cessation prior to undergoing major colonic resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the fourth most common malignancy and the second most common cause of cancer death globally [1]. Right-sided colon cancer accounts for approximately 30% of all large bowel cancers and commonly treated by right hemicolectomy (RH) [2]. The tobacco epidemic is one of the biggest public health issues and accounts for the death of approximately 8 million people worldwide [3]. Latest prevalence estimates that 49% of men and 8% of women smoke in low- and middle-income countries with a ratio that varies slightly in higher income nations (37% for men and 21% for women). Despite active public health interventions such as negative advertising, tax increase and age restrictions, smoking remains a significant global problem with the highest percentage of smokers aged between 45 and 64 years old [4]. With high prevalence of smokers and increasing rates of colon cancer, clinicians and surgeons are more likely to encounter patients with newly diagnosed right colon cancer who are long-term smokers.
Most surgical literature strongly supports the negative association of long-term preoperative smoking and increased complications rates in both gastrointestinal (GI) and non-GI surgery [5,6,7,8,9,10,11,12,13]. These studies are consistent in demonstrating higher rates of post-operative morbidity such as wound infections, pulmonary complications and increased anastomotic leaks (ALs). Consequently, this translates to a substantial financial cost to the health system [14,15,16]. However, many studies assessing the impact of preoperative smoking on GI surgery are quite heterogenous consisting of a variety of operation types and pathologies, therein reducing its overall specificity [6,7,8]. To our knowledge, there is no data assessing the direct impact of preoperative smoking on RH.
The aim of this study was to use the American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) database to quantify the complication risk profile in smokers undergoing a RH for colon cancer, compared with non-smokers. This would support clinicians and surgeons to objectively evaluate what the negative influence of smoking is on post major colonic resection and inform patients of their associated risks compared to non-smokers.
Methods
Study population
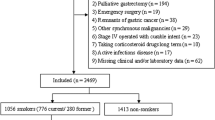
The ACS-NSQIP is a national validated database which prospectively collects global data from more than 600 hospitals worldwide (including our own institution) with over 200 targeted validated outcome variables. Sampling strategy, data abstraction and outcomes recorded using this database have been previously validated [17, 18]. This database was queried for patients with a primary postoperative diagnosis of right sided colon cancer between 2016 and 2019 based on the International Classification of Disease, Ninth (ICD-9) and Tenth (ICD-10) revision codes. Only those with pathologic confirmation of malignant neoplasm of the caecum, ascending colon, hepatic flexure and proximal transverse colon were included. The primary diagnosis was then cross-referenced with the Current Procedural Terminology (CPT) codes 44204 (laparoscopy colectomy partial w/anastomosis), 44205 (laparoscopy, surgical; colectomy, partial, with removal of terminal ileum with ileocolostomy), 44140 (colectomy partial w/anastomosis) and 44160 (colectomy, partial, with removal of terminal ileum with ileocolostomy). Patients who underwent either a laparoscopic or robotic procedure were coded as a minimally invasive procedure (MIP). If patients were coded to having a MIP with an unplanned conversion to open, then this was coded as a “MIP converted to open.” Open procedures were classified as a separate group. Due to the use of de-identified patient data only, this study is considered non-human subjects research and no ethics is required.
Inclusion and exclusion criteria
To allow for a more homogenous and meaningful analysis of data, we excluded all patients who underwent emergency RH, as well as those with a preoperative American Society of Anesthesiologists (ASA) class of 5 (moribund), systemic inflammatory response syndrome (SIRS), sepsis or septic shock, acute renal failure, ventilator dependence and pneumonia.
Predictor variables
Patients were grouped into smokers and non-smokers defined as smoking cigarettes within 12 months prior to surgery, as defined by the NSQIP database. Standard reported patient demographics as provided by the NSQIP database includes age, sex, race and ethnicity. Patient clinical characteristics included body mass index (BMI), ASA classification, preoperative weight loss > 10% of total weight, functional status and chronic steroid use. Preoperative medical comorbidity variables included insulin-dependent (IDDM) or non-insulin-dependent diabetes mellitus (NIDDM), dyspnoea, chronic obstructive pulmonary disease (COPD), hypertension and disseminated cancer. Surgical characteristics of interest included operative time, procedure type (open, laparoscopic, robotic) and wound classification.
Outcomes
Outcomes evaluated included complications that occurred within 30 days of surgery as validated by the NSQIP database: SSI (including superficial, deep or organ space types), wound dehiscence, sepsis, Clostridium difficile (C. diff) infection, urinary tract infection (UTI), pneumonia, reintubation, deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, myocardial infarction (MI), renal failure or injury, bleeding requiring > 4 unit transfusion, return to the operating room, prolonged length of stay (LOS) defined by patients in 4th quartile for LOS (8 days), hospital readmission, anastomotic leak (AL), prolonged NPO/nasogastric tube > 48 h (ileus), or all-cause mortality.
Composite outcomes of interest evaluated included:
-
1.
Wound complications (superficial and/or deep SSI, and wound dehiscence)
-
2.
Surgical complications (wound complications, organ space infection, unplanned return to operating room, bleeding requiring transfusion and AL)
-
3.
Medical complications (pneumonia, PE, failure to wean from ventilator and reintubation, progressive or acute renal insufficiency (creatinine > 2 mg/dL), MI, cardiac arrest with or without cardiopulmonary resuscitation)
-
4.
Primary pulmonary complications (pneumonia, reintubation and failure to wean from ventilator)
-
5.
Embolic complications (DVT/PE)
-
6.
Renal complications [acute or progressive renal failure (creatinine > 2 mg/dL)]
-
7.
Septic complications (sepsis or septic shock). Sepsis was evaluated separately as its aetiology could have been either medical or surgical without any clear distinction from the NSQIP database.
Statistical analysis
Propensity score matching was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Propensity scores were calculated based on the patient’s clinical characteristics and preoperative medical comorbidities as previously listed (age, sex, race, BMI, ASA classification, preoperative weight loss > 10% of total weight, functional status, chronic steroid use, diabetes mellitus, dyspnoea, chronic obstructive pulmonary disease (COPD), hypertension and disseminated cancer). 2:1 matching was used with a maximum absolute difference of 0.05 between propensity scores, with matching optimized for number of matches. Normally distributed continuous variables were analysed using two-sample Student’s t tests, whilst categorical variables were assessed with chi-square testing. Odds ratios (OR) of the composite and individual outcomes of interest were calculated using both univariate logistic analysis and multivariate regression (age, sex, functional status, diabetes, COPD, congestive heart failure, medicated hypertension, chronic steroid use, BMI and ASA class were considered within the model). Observations with missing values were censored from analyses.
Results
Demographics
The original population had 17,033 patients who underwent elective RH for colon cancer with 1,884 (11.1%) who identified as smokers (Table 1). There was an almost equal distribution of male to female smokers (51.3% vs 48.7%, p = 0.005). Smokers were significantly younger than non-smokers (64 years vs 70 years, p < 0.001). The mean operation time was 155 min (SD = 70 min) with smokers yielding a significantly longer operative time of 4 min (158 vs 154, p = 0.005). Smokers also had a significantly longer LOS of 0.25 days compared to non-smokers (5.2 days vs 4.95 days, p = 0.001). The most common ethnicity group was Caucasians (68.4%), followed by African Americans (9.6%) and Asians (3.6%). There were 3,650 (21.4%) patients with diabetes mellitus of which 2,495 (14.6%) were NIDDM and 1,155 (6.8%) IDDM. The mean BMI was 28.8 kg/m2 (SD = 6.9), with smokers having a significantly lower BMI distribution compared to non-smokers (p < 0.001). Most patients (60.1%) had a preoperative ASA score of 3, though smokers were significantly more likely to have a higher ASA grade (p < 0.001). Most procedures were performed as a MIP in 13,187 cases (77.4%), followed by planned open surgery 2,743 (16.1%), 1,103 (6.5%) started as a MIP then converted to open.
Following propensity score matching, 5,652 patients who underwent elective RH for colon cancer were selected with 1,884 (33.3%) who identified as smokers (Table 1). Smokers remained significantly younger than non-smokers (64 years vs 66 years, p < 0.001). There was no significant difference between the mean operation time for smokers and non-smokers (p = 0.734), with an overall mean of 158 min (SD = 74 min). In relation to preoperative comorbidities, there were 1,043 (18.5%) patients with diabetes mellitus; of these, 726 (12.9%) were NIDDM and 317 (5.6%) IDDM, with no significant difference between smokers and non-smokers (p = 0.199). COPD remained more significant in the smokers than non-smokers (14.3% vs 10.6, p < 0.001). The mean BMI was 28.8 kg/m2 (SD = 6.9). Most patients (63.0%) had a preoperative ASA score of 3 with no difference in ASA distribution between smokers and non-smokers (p = 0.367). Most procedures were performed as a MIP in 4,233 cases (74.9%), followed by planned open surgery 1,025 (18.1%), and 394 (7.0%) started as a MIP then converted to open.
Outcomes
Analysis of 30-day outcomes of individual complications is summarised in Table 2. Of clinical significance, smokers were found to have a higher rate of organ space infection (4.1% vs 3.1%, p = 0.034), acute renal failure (0.6% vs 0.1%, p = 0.001), unplanned return to the operating room (4.8% vs 3.7%, p = 0.045) and anastomotic leak (3.5% vs 2.1%, p = 0.005). The rate of AL was observed in 144/5652 (2.5%) patients. Higher proportion of wound-related complications (superficial SSI, deep SSI and wound dehiscence) was observed in smokers but of no clinical significance. Similarly, also not significant, there was a trend towards higher risk of pneumonia, reintubation and sepsis in smokers.
After adjusting for patient comorbidities and operative approach, multivariable analysis for selected outcomes and composite outcomes demonstrated smoking to be an independent risk factor for wound complications (OR 1.32, 95% CI 1.03–1.71, p = 0.032), primary pulmonary complications (OR 1.50, 95% 1.06–2.13, p = 0.024) and AL (OR 1.66, 95% CI 1.19–2.31, p = 0.003). Major medical complications (OR 1.15, 95% CI 0.97–1.36, p = 0.114), surgical complications (OR 1.09, 95% CI 0.93–1.29, p = 0.282), embolic complications (OR 1.27, 95% CI 0.77–2.07, p = 0.797), renal (OR 1.72, 95% CI 0.92–3.22, p = 0.091) and sepsis (OR 1.19, 95% CI 0.87–1.63, p = 0.284) trended towards a higher risk for smokers but did not reach statistical significance (Table 3).
Discussion
Clinicians and surgeons will encounter patients with a newly diagnosed colon cancer who are long-term smokers, often at a relatively young age. Indeed, we identified that smokers were significantly younger than non-smokers by an average of 6 years (p < 0.001) in the original population. This can be explained by the fact that smokers have an estimated higher risk of developing colorectal cancer of 20 to 60% [19]. Studies that have evaluated the complication risk profile of chronic smokers undergoing GI surgery have reported an increased rate of impaired wound healing, increased infection and cardiopulmonary complication rates [6, 7, 9]. This has a detrimental impact on patients’ wellbeing and quality of life. In addition, this also poses a financial burden on health care resources. It is therefore beneficial to risk stratify long-term smokers undergoing surgery and mitigate those risks in the preoperative period. Our analysis of the NSQIP database on elective RH for bowel cancer remains consistent with current literature demonstrating increased wound and pulmonary complications, as well as AL rates amongst smokers compared with non-smokers. The challenge therein lies in the effective perioperative management in this group of patients, including national pre-operative smoking cessation programmes that could potentially mitigate their complication risk.
Using the NSQIP database, Sharma and colleagues analysed over 47,000 patients who underwent a colonic resection for benign (diverticular disease and inflammatory bowel disease) and malignant (colorectal cancer) pathologies, demonstrating almost a 1.5-fold increase in morbidity and mortality amongst smokers [6]. Similarly, Brajcich and colleagues reported higher rates of death and serious postoperative morbidity amongst chronic smokers in patients undergoing various GI procedures (colorectal, pancreatic, gastric or hepatic procedures) [7]. Both studies included large sample size, but the heterogeneity of selected patients potentially masks the real impact of smoking on specific operation types, thus limiting the true understanding of its explicit procedure-related risk. In comparison, our study offers a more homogenous picture, therefore reducing variability and heterogeneity, whilst adding clearer risk stratification for patients undergoing RH.
Wound complications, in particular SSIs, are the third most reported type of healthcare-associated infections. Their management is labour intensive and is associated with prolonged LOS and an additional economic burden [20]. Long-term smoking increases the likelihood of SSIs and wound dehiscence. A large systematic review and meta-analysis assessing wound complications in smokers across a range of surgical specialties reported a twofold increase in adjusted OR for healing delay and wound dehiscence, 1.8-fold increase in SSI and 2.3-fold increase in overall wound complication rates [21]. Brajcich et al. and Sharma et al. similarly reported approximately a 1.3-fold increase in wound complications of clinical significance [6, 7]. In our study, smokers also had a 1.3-fold increased risk of wound complications, confirming its negative impact that is in line with current literature. Wound complications are also influenced by the use of long-term steroids [22]. Ismael et al. analysed over 600,000 patients from the NSQIP database and reported in those patients with steroid use, increased SSI rates from 2.9 to 5.0% (OR = 1.724), increased deep SSIs from 0.8 to 1.8% (OR = 2.353), and wound dehiscence increased risk of 2- to threefold (OR = 3.338). In our study, 3.0% of smokers were on long-term steroids compared with 3.5% in non-smokers. Interestingly, our results confirmed that the negative effect of smoking still outweighed the established risk of steroids on healing.
This study set out to stratify the risk of medical-related complications as a separate outcome in long-term smokers. The pre-anaesthesia co-morbidity (ASA) score has been shown to be a reliable independent predictor of medical complications and mortality following surgery [23]. In our cohort of patients, most smokers were either ASA 3 or above when compared to non-smokers (64.4% vs 62.2%, p = 0.367). The higher proportion of ASA 3 and above in smokers is most probably related to their associated medical comorbidities because of long-term smoking, which include cardiopulmonary-related disease. Lifelong smokers have a 50% probability of developing COPD during their lifetime and studies have proven that COPD is an independent risk factor for pulmonary complications [24, 25]. Unsurprisingly, the proportion of patients suffering from COPD amongst smokers was also higher than non-smokers (14.3% vs 10.6%). Adjusting for patient comorbidities, the risk of specific medical-related complications was higher (OR = 1.15, 95% CI 0.97–1.36, p = 0.114) in long-term smokers but not of clinical significance. Furthermore, we also identified that the rate of pneumonia was higher in smokers (2.20% vs 1.50%, p = 0.085) and smokers had a 1.5-fold increase in primary pulmonary complications compared to non-smokers. Prolonged ventilation is a recognised risk factor for increased postoperative pulmonary complications [25]. As such, we identified a trend towards higher reintubation (and therefore prolonged ventilation) rate in smokers (1.5% versus 1.0%, p = 0.101) when compared to non-smokers but of not clinical significance. We believe that larger sample size would eventually confirm those trends.
Analysis of sepsis was conducted as a separate outcome due to its aetiology (either medical or surgical) being indistinguishable from the NSQIP database. This strategy was different to similar studies and provides a more precise outcome measure of complication profile in smokers. The likelihood of sepsis was 1.2-fold higher in smokers compared to non-smokers, but this was not clinically significant. We also know that patients returning to theatre, especially for AL, are most likely to present with symptoms of sepsis that is associated with longer length of hospital stay and trends towards a higher mortality rate [26]. The average LOS for those patients is approximately 75% greater than for most other conditions and dramatically increases with sepsis severity [27]. In our study, we identified a higher rate of return to operating room (4.8% vs 3.7%, p = 0.045), organ space infection (4.1% vs 3.1%, p = 0.034) and AL (3.5% vs 2.1%, p < 0.005) amongst smokers that also explains the increased likelihood of sepsis in this group of patients. AL is probably the most serious and feared complication specific to colorectal surgery as it is associated with significant morbidity and mortality [28, 29]. The literature reports a risk of AL post RH between 3 and 8% [30, 31]. From this cohort of selected patients, we found a lower overall rate of only 2.6%. This difference is likely attributed to our exclusion of emergency procedures and other unfavourable factors, such as preoperative sepsis and moribund patients not expected to survive without surgery (ASA 5 score), that could have had positively influenced outcome. Despite these exclusions, we identified that smokers had a 1.6-fold increased risk of AL compared to non-smokers. Sorensen and colleagues reported similar threefold increase in smokers after colorectal surgery, much higher due to the inclusion of left-sided colonic resections [32]. Once again, this outlines the importance of preoperative assessment and early smoking cessation to minimise this risk of AL.
As clinicians, we must acknowledge the increased risk of medical and surgical complications in smokers, as highlighted by this study. Preoperative risk stratification will enable health care professionals in minimising those risks, focussing on prevention rather than curative measures. Strategies that may be of benefit include preoperative smoking cessation programs that have demonstrated positive effects on post-surgical outcome, however not supported by others [33,34,35,36]. These inconsistencies likely reflect the differences in duration of preoperative smoking cessation, variability in types of surgery selected, and differing endpoints. For instance, the minimum duration of smoking cessation may vary between 2 and 8 weeks depending on the type of intended surgical procedure [37,38,39,40]. Additionally, extended timing between diagnosis and planned surgery may not always be possible, especially when dealing with colonic malignancies. Unsurprisingly, Sorensen and colleagues demonstrated that shorter-term smoking cessation failed to improve tissue and wound healing, as well as complication profile [33]. Extended smoking cessation schemes are therefore paramount for reducing the risk of post-operative complications and should remain an important target for quality and improvement [7]. The introduction of Enhanced Recovery After Surgery (ERAS) protocol specific to colorectal surgery has substantially improved post-operative outcomes in patients undergoing colorectal procedures [41]. Additional modifications of this ERAS programme for smokers may be considered, for example, with the introduction of a more aggressive approach to chest physiotherapy to minimise postoperative pulmonary complications and longer broad-spectrum antibiotics use, but also to minimise the risk of wound and septic complications. The potential success of these proposed strategies is probably best answered by observational or well-conducted randomised studies.
We acknowledge certain limitations in this study. For instance, definition of smoking as per the variable definition from the NSQIP database includes current smoking as a history of smoking within a year of surgery. This means that one who may have quit smoking several months before surgery would still be classified as a smoker. Secondly, there is continuous debate on the risk of AL based on the surgical technique (stapled versus handsewn) in the elective setting [42, 43]. This study does not account for the surgical technique nor surgeon experience that could have influenced the outcome of AL, or other complications. Thirdly, the use of preoperative antibiotics at induction is standard practice in gastrointestinal surgery. We assume that patients would have been administered the appropriate regimen as per local hospital protocol. However, this variable is unaccounted for in this study. The strength of our study is that 30-day outcomes were retrospectively reviewed from a large-scale prospectively maintained cohort of patients where all pre- and post-operative factors are objectively collected in a consistent and validated manner, by NSQIP hospital data coordinators. Additionally, we have used strict inclusion and exclusion criteria of the medical and surgical composite outcomes.
In conclusion, this study highlights important issues surrounding complications associated with smoking, post RH for bowel cancer. Smoking is an independent risk factor of wound complications, pulmonary complications, unplanned return to operating room, sepsis and AL. This study supports and supplements the current literature by providing a more in-depth analysis in the risk profile of long-term smokers, using composite outcomes that are clinically relevant to patients and clinicians. Surgeons who counsel patients undergoing RH should emphasize these increased risk of complications prior to elective surgery.
Change history
27 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00423-022-02528-2
References
Rawla P, Sunkara T, Barsouk A (2019) Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 14(2):89–103
Gomez D, Dalal Z, Raw E, Roberts C, Lyndon PJ (2004) Anatomical distribution of colorectal cancer over a 10 year period in a district general hospital: is there a true “rightward shift”? Postgrad Med J 80(949):667–669
Collaborators GBDT (2021) Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397(10292):2337–2360
Golechha M (2016) Health Promotion methods for smoking prevention and cessation: a comprehensive review of effectiveness and the way forward. Int J Prev Med 7:7
Sorensen LT, Horby J, Friis E, Pilsgaard B, Jorgensen T (2002) Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol 28(8):815–820
Sharma A, Deeb AP, Iannuzzi JC, Rickles AS, Monson JR, Fleming FJ (2013) Tobacco smoking and postoperative outcomes after colorectal surgery. Ann Surg 258(2):296–300
Brajcich BC, Yuce TK, Merkow RP, Bilimoria KY, McGee MF, Zhan T et al (2021) Association of preoperative smoking with complications following major gastrointestinal surgery. Am J Surg 223(2):312–317
Gajdos C, Hawn MT, Campagna EJ, Henderson WG, Singh JA, Houston T (2012) Adverse effects of smoking on postoperative outcomes in cancer patients. Ann Surg Oncol 19(5):1430–1438
Inoue Y, Katoh T, Masuda S, Lu X, Koga T, Sadohara T et al (2020) Perioperative complications of abdominal surgery in smokers. J Anesth 34(5):712–718
Quilichini O, Barrou J, Bannier M, Rua S, Van Troy A, Sabiani L et al (2021) Mastectomy with immediate breast reconstruction: results of a mono-centric 4-years cohort. Ann Med Surg (Lond) 61:172–179
Pastoriza J, McNelis J, Parsikia A, Lewis E, Ward M, Marini CP et al (2021) Predictive factors for surgical site infections in patients undergoing surgery for breast carcinoma. Am Surg 87(1):68–76
Bhama AR, Batool F, Collins SD, Ferraro J, Cleary RK (2017) Risk factors for postoperative complications following diverting loop ileostomy takedown. J Gastrointest Surg 21(12):2048–2055
Quan H, Ouyang L, Zhou H, Ouyang Y, Xiao H (2019) The effect of preoperative smoking cessation and smoking dose on postoperative complications following radical gastrectomy for gastric cancer: a retrospective study of 2469 patients. World J Surg Oncol 17(1):61
Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr (2004) Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 199(4):531–537
Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA (2003) Complications and costs after high-risk surgery: where should we focus quality improvement initiatives? J Am Coll Surg 196(5):671–678
Zhan C, Miller MR (2003) Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 290(14):1868–1874
Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, et al (1998) The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 228(4):491–507
Khuri SF (2005) The NSQIP: a new frontier in surgery. Surgery 138(5):837–843
Hannan LM, Jacobs EJ, Thun MJ (2009) The association between cigarette smoking and risk of colorectal cancer in a large prospective cohort from the United States. Cancer Epidemiol Biomarkers Prev 18(12):3362–3367
Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C (2017) Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 96(1):1–15
Sorensen LT (2012) Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg. 147(4):373–83
Wang AS, Armstrong EJ, Armstrong AW (2013) Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg 206(3):410–417
Hackett NJ, De Oliveira GS, Jain UK, Kim JY (2015) ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 18:184–190
Laniado-Laborin R (2009) Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health. 6(1):209–24
Fernandez-Bustamante A, Frendl G, Sprung J, Kor DJ, Subramaniam B, Martinez Ruiz R et al (2017) Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg 152(2):157–166
Alroumi F, Abdul Azim A, Kergo R, Lei Y, Dargin J (2018) The impact of smoking on patient outcomes in severe sepsis and septic shock. J Intensive Care 6:42
Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A (2011) Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 62:1–8
European Society of Coloproctology Collaborating G (2020) Predictors for anastomotic leak, postoperative complications, and mortality after right colectomy for cancer: results from an international snapshot audit. Dis Colon Rectum. 63(5):606–18
Moran BJ, Heald RJ (2001) Risk factors for, and management of anastomotic leakage in rectal surgery. Colorectal Dis 3(2):135–137
Ng CW, Prabhakaran S, Chakraborty J, Lutton N, Gourlas P, Gillespie C et al (2020) Rate of anastomotic leak following right hemicolectomy by general surgical trainees. Int J Colorectal Dis 35(12):2339–2346
Frasson M, Granero-Castro P, Ramos Rodriguez JL, Flor-Lorente B, Braithwaite M, Marti Martinez E et al (2016) Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: results from a prospective, multicentric study of 1102 patients. Int J Colorectal Dis 31(1):105–114
Sorensen LT, Jorgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jorgensen P (1999) Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg 86(7):927–931
Sorensen LT, Jorgensen T (2003) Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Colorectal Dis 5(4):347–352
Rodriguez M, Gomez-Hernandez MT, Novoa N, Jimenez MF, Aranda JL, Varela G (2017) Refraining from smoking shortly before lobectomy has no influence on the risk of pulmonary complications: a case-control study on a matched population. Eur J Cardiothorac Surg 51(3):498–503
Myers K, Hajek P, Hinds C, McRobbie H (2011) Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med 171(11):983–989
Thomsen T, Villebro N, Moller AM (2010) Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. (7):CD002294
Jung KH, Kim SM, Choi MG, Lee JH, Noh JH, Sohn TS et al (2015) Preoperative smoking cessation can reduce postoperative complications in gastric cancer surgery. Gastric Cancer 18(4):683–690
Kuri M, Nakagawa M, Tanaka H, Hasuo S, Kishi Y (2005) Determination of the duration of preoperative smoking cessation to improve wound healing after head and neck surgery. Anesthesiology 102(5):892–896
Lindstrom D, Sadr Azodi O, Wladis A, Tonnesen H, Linder S, Nasell H et al (2008) Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 248(5):739–745
Moller AM, Villebro N, Pedersen T, Tonnesen H (2002) Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 359(9301):114–117
Ban KA, Berian JR, Ko CY (2019) Does implementation of enhanced recovery after surgery (ERAS) protocols in colorectal surgery improve patient outcomes? Clin Colon Rectal Surg 32(2):109–113
Espin E, Vallribera F, Kreisler E, Biondo S (2020) Clinical impact of leakage in patients with handsewn vs stapled anastomosis after right hemicolectomy: a retrospective study. Colorectal Dis 22(10):1286–1292
Abounozha S, Kheder A, Alshahri T, Ibrahim R (2020) Best evidence topic: Is ileocolic anastomotic leak rate higher in handsewn or stapler’s anastomosis? Ann Med Surg (Lond) 60:619–622
Acknowledgements
We would like to extend our acknowledgement to Mingjuan Zeng, NSQIP hospital data coordinator, and Peter Geelan-Small, statistician, University of New South Wales, Data Analytical Centre.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
The authors contributed to the conception and design of the manuscript, revised it critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badiani, S., Diab, J., Woodford, E. et al. Impact of preoperative smoking on patients undergoing right hemicolectomies for colon cancer. Langenbecks Arch Surg 407, 2001–2009 (2022). https://doi.org/10.1007/s00423-022-02486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02486-9