Abstract
Purpose
The aim was to evaluate the oncological outcomes and the prognostic factors following pelvic exenteration (PE) in cT4 and fixed cT3 stage primary rectal adenocarcinoma and to study the impact of consolidation chemotherapy following neoadjuvant concurrent chemoradiotherapy (NACRT).
Methods
A retrospective analysis of a prospectively maintained database of PE from 2013 to 2018.
Results
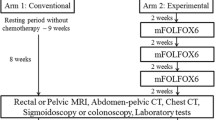
Out of 2900 colorectal resections, there were 131 pelvic exenterations that were performed, and 100 of these patients had undergone exenteration for primary rectal adenocarcinoma. Of these 100 patients, there were 81 patients who had received NACRT followed by surgery, 50 of whom who had received consolidation chemotherapy and 31 who had undergone surgery without consolidation chemotherapy. R0 resection was achieved in 90% cases. At a median follow-up of 32 months, 2-year disease free survival was 61.8% and estimated 5-year overall survival was 62%. The incidence of distant metastases was 44% vs. 19% (p = 0.023), and the 2-year distant recurrence-free survival was 58% vs. 89% (p = 0.025), respectively, in the ‘consolidation chemotherapy group’ and the ‘no chemotherapy group’. The poorly differentiated grade of tumours, presence of lympho-vascular-invasion, consolidation chemotherapy, and disease recurrence were all found to affect the survival.
Conclusion
PE with R0 resection achieves excellent survival rates in cT4 and fixed cT3 stage primary rectal adenocarcinoma. The distant recurrence rate may not be altered by consolidation chemotherapy in the subset of high-risk patients. However, further research on consolidation chemotherapy following NACRT in cT4 and fixed cT3 stage primary rectal adenocarcinoma will give a definite answer in the future.

Similar content being viewed by others
Data availability
Data will be provided on request.
Code availability
Not applicable.
References
GLOBOCAN 2018.
Loomans-Kropp HA, Umar A (2019) Increasing incidence of colorectal cancer in young adults. J Cancer Epidemiol 2019:9841295
Badwe RA, Dikshit R, Laversanne M, Bray F (2014) Cancer incidence trends in India. Jpn J Clin Oncol 44:401–407
Patil PS, Saklani A, Gambhire P, Mehta S, Engineer R, DeSouza A et al (2017) Colorectal cancer in India: an audit from a tertiary center in a low prevalence area. Indian J Surg Oncol 8:484–490
Haleshappa RA, Rao SA, Garg S, Kuntegowdanahalli CL, Kanakasetty GB, Dasappa L (2017) Is colorectal cancer in young (<40 years) different from those in the elderly (>40 years): experience from a regional care center. Indian J Med Paediatr Oncol 38(4):466–470
Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis PJ, Hugen N, Nagtegaal ID, de Wilt JHW, Verhoeven RHA (2018) An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer 143(11):2758–2766
PelvEx Collaborative (2019) Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg 269(2):315–321
Benitez Majano S, Di Girolamo C, Rachet B, Maringe C, Guren MG, Glimelius B et al (2019) Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. Lancet Oncol 20(1):74–87
National Comprehensive Cancer Network. Rectal cancer (Version 3. 2020). https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf Accessed May 6, 2020.
Quyn AJ, Austin KK, Young JM, Badgery-Parker T, Masya LM, Roberts R et al (2016) Outcomes of pelvic exenteration for locally advanced primary rectal cancer: overall survival and quality of life. Eur J Surg Oncol 42(6):823–828
Yang TX, Morris DL, Chua TC (2013) Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum 56(4):519–531
Sobin LH, Gospodarowicz M, Wittekind C (2009) TNM Classification of Malignant Tumours. Seventh Edition. UICC International Union Against Cancer. New York: Wiley-Blackwell
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Platt E, Dovell G, Smolarek S (2018) Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Tech Coloproctol 22(11):835–845
Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S (2019) Early-onset colorectal cancer in young individuals. Mol Oncol 13(2):109–131
Rana N, Chakravarthy AB, Kachnic LA (2017) Neoadjuvant treatment for locally advanced rectal cancer: new concepts in clinical trial design. Curr Treat Options in Oncol 18:13
De Caluwé L, Van Nieuwenhove Y, Ceelen WP (2013) Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev (2):CD006041
Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E, Fritz E, Hieber U, Lindemann HW, Grunewald M, Kremers S, Constantin C, Hipp M, Hartung G, Gencer D, Kienle P, Burkholder I, Hochhaus A (2012) Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 13(6):579–588
Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, Michalski W, Olędzki J, Kuśnierz J, Zając L, Bednarczyk M, Szczepkowski M, Tarnowski W, Kosakowska E, Zwoliński J, Winiarek M, Wiśniowska K, Partycki M, Bęczkowska K, Polkowski W, Styliński R, Wierzbicki R, Bury P, Jankiewicz M, Paprota K, Lewicka M, Ciseł B, Skórzewska M, Mielko J, Bębenek M, Maciejczyk A, Kapturkiewicz B, Dybko A, Hajac Ł, Wojnar A, Leśniak T, Zygulska J, Jantner D, Chudyba E, Zegarski W, Las-Jankowska M, Jankowski M, Kołodziejski L, Radkowski A, Żelazowska-Omiotek U, Czeremszyńska B, Kępka L, Kolb-Sielecki J, Toczko Z, Fedorowicz Z, Dziki A, Danek A, Nawrocki G, Sopyło R, Markiewicz W, Kędzierawski P, Wydmański J, Albiński J, Banaś R, Chmielowska E, Bal W, Baszczyk-Mnich J, Bialas M, Borowiec T, Bujko M, Cencelewicz A, Chomik K, Chwaliński M, Ciepela I, Dupla D, Florek A, Górnicki A, Jeziorski K, Józwicki W, Kobiela J, Koda M, Kołodziej P, Kruszewski P, Kryj M, Kuciel-Lisiecka G, Kwiatkowski R, Lachowski A, Liszka-Dalecki P, Majewski A, Majewski W, Majsak T, Maka D, Malka M, Mazurkiewicz A, Morawiec J, Nogal E, Olejniczak M, Olkowski D, Ostrowska-Cichocka K, Pietruszka M, Piotrkowski G, Plewicka M, Porzuczek-Zuziak D, Reszke J, Rychter A, Sadowski J, Salata A, Serkies K, Srutek E, Szóstak B, Tuziak T, Tyralik D, Skoczylas J, Wachua E, Wandzel P, Winkler-Spytkowska B, Wojtasik P, Wroński K, Zemal M, Zygulski I (2016) Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 27(5):834–842
Ciseł B, Pietrzak L, Michalski W, Wyrwicz L, Rutkowski A, Kosakowska E, Cencelewicz A, Spałek M, Polkowski W, Jankiewicz M, Styliński R, Bębenek M, Kapturkiewicz B, Maciejczyk A, Sadowski J, Zygulska J, Zegarski W, Jankowski M, Las-Jankowska M, Toczko Z, Żelazowska-Omiotek U, Kępka L, Socha J, Wasilewska-Tesluk E, Markiewicz W, Kładny J, Majewski A, Kapuściński W, Suwiński R, Bujko K, Polish Colorectal Study Group (2019) Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol 30(8):1298–1303
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, Ledermann J, Sebag-Montefiore D (2014) Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 25:1356–1362
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, van Laethem J, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L, EORTC Radiation Oncology Group (2014) EORTC Radiation Oncology Group. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD (2015) Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol 26(8):1722–1728
Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Schmiegel W, Jacobasch L, Weitz J, Folprecht G, Schlenska-Lange A, Flentje M, Germer CT, Grützmann R, Schwarzbach M, Paolucci V, Bechstein WO, Friede T, Ghadimi M, Hofheinz RD, Rödel C, on behalf of the German Rectal Cancer Study Group (2019) Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol 37(34):3212–3222
Sclafani F, Brown G, Cunningham D, Rao S, Tekkis P, Tait D, Morano F, Baratelli C, Kalaitzaki E, Rasheed S, Watkins D, Starling N, Wotherspoon A, Chau I (2017) Systemic chemotherapy as salvage treatment for locally advanced rectal cancer patients who fail to respond to standard neoadjuvant chemoradiotherapy. Oncologist. 22(6):728–736
Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K, Timing of Rectal Cancer Response to Chemoradiation Consortium (2015) Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 16(8):957–966
Marco MR, Zhou L, Patil S, Marcet JE, Varma MG, Oommen S, Cataldo PA, Hunt SR, Kumar A, Herzig DO, Fichera A, Polite BN, Hyman NH, Ternent CA, Stamos MJ, Pigazzi A, Dietz D, Yakunina Y, Pelossof R, Garcia-Aguilar J, Timing of Rectal Cancer Response to Chemoradiation Consortium (2018) Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum 61(10):1146–1155
Ostwal V, Engineer R, Ramaswamy A, Sahu A, Zanwar S, Arya S, Chopra S, Bal M, Patil P, Desouza A, Saklani A (2016) Surgical outcomes of post chemoradiotherapy unresectable locally advanced rectal cancers improve with interim chemotherapy, is FOLFIRINOX better than CAPOX? J Gastrointest Oncol 7(6):958–967
Denost Q, Kontovounisios C, Rasheed S, Chevalier R, Brasio R, Capdepont M, Rullier E, Tekkis PP (2017) Individualizing surgical treatment based on tumour response following neoadjuvant therapy in T4 primary rectal cancer. Eur J Surg Oncol 43(1):92–99
Verma K, Engineer R, Ostwal V, Kumar S, Arya S, Desouza AL, Saklani AP (2018) Persistent involvement of anterior mesorectal fascia in carcinoma rectum - extended resection of rectum vs total pelvic exenteration: results from a single-centre retrospective study. Color Dis 20(12):1070–1077
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR et al (2015) Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 15:767
Kim CW, Kang BM, Kim IY, Kim JY, Park SJ, Park WC, Bae KB, Bae BN, Baek SK, Baik SH, Son GM, Lee YS, Lee SH (2018) Korean Society of Coloproctology (KSCP) trial of Consolidation Chemotherapy for Locally advanced mid or low rectal cancer after neoadjuvant concurrent chemoradiotherapy: a multicenter, randomized controlled trial (KONCLUDE). BMC Cancer 18(1):538
Bahadoer RR, Dijkstra EA, van Etten B, et al (2020) RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 7:S1470-2045(20)30555-6
Conroy T, Lamfichekh N, Etienne PL et al (2020) Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. Proc Am Soc Clin Oncol 38:4007 (abstr)
Georgiou PA, Tekkis PP, Constantinides VA, Patel U, Goldin RD, Darzi AW (2013) Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Cancer 49(1):72e81
Waters PS, Peacock O, Warrier SK, Wakeman C, Eglinton T, Lynch AC, Frizelle FA, Heriot AG, McCormick J (2019) Evolution of pelvic exenteration surgery- resectional trends and survival outcomes over three decades. Eur J Surg Oncol 45(12):2325–2333
Bhangu A, Ali SM, Brown G, Nicholls RJ, Tekkis P (2014) Indications and outcome of pelvic exenteration for locally advanced primary and recurrent rectal cancer. Ann Surg 259(2):315–322
Kusters M, Austin KKS, Solomon MJ, Lee PJ, Nieuwenhuijzen GAP, Rutten HJT (2015) Survival after pelvic exenteration for T4 rectal cancer. Br J Surg 102:125–131
Radwan RW, Jones HG, Rawat N, Davies M, Evans MD, Harris DA, Beynon J, Swansea Pelvic Oncology Group, McGregor AD, Morgan AR, Freites O, Patel B, Askill C, Rowley C, Pudney D, Hatcher O, Bose P, Fenn N, Lucas MG, Khot U, Chandrasekaran TV, Carr ND, Gwynne S, Drew P, Phan MD (2015) Determinants of survival following pelvic exenteration for primary rectal cancer. Br J Surg 102:1278–1284
PelvEx Collaborative (2019) Changing outcomes following pelvic exenteration for locally advanced and recurrent rectal cancer. BJS Open 3(4):516–520
Steffens D, Solomon MJ, Young JM, Koh C, Venchiarutti RL, Lee P, Austin K (2018) Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open 2(5):328–335
Author information
Authors and Affiliations
Contributions
NK, AD, and AS did the study conception and design. NK, SS, and KV acquired the data. NK, AD, VO, AR, RE, and AS performed the analysis and interpretation of data. NK, AD, VO, SS, KV, AR, RE, and AS drafted the manuscript. NK, AD, VO, RE, and AS are responsible for the critical revision of manuscript.
Corresponding author
Ethics declarations
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. No institutional research committee approval was required as this was a retrospective study.
Consent to participate
Written informed consent was obtained from the patient.
Consent for publication
Written informed consent for publication of their clinical details was obtained from the patient.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, N.A., Desouza, A., Ostwal, V. et al. Outcomes of exenteration in cT4 and fixed cT3 stage primary rectal adenocarcinoma: a subgroup analysis of consolidation chemotherapy following neoadjuvant concurrent chemoradiotherapy. Langenbecks Arch Surg 406, 821–831 (2021). https://doi.org/10.1007/s00423-021-02143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02143-7