Abstract
Background
In locally advanced rectal cancer (LARC), the optimal sequence of neoadjuvant chemotherapy in relation to neoadjuvant chemoradiotherapy and before total mesorectal excision is unknown.
Methods
A total of 426 LARC patients, treated with neoadjuvant chemoradiotherapy followed by total mesorectal excision, between January 2010 and December 2018, were studied retrospectively. Patients were divided into induction and consolidation chemotherapy groups. Overall, disease-free, locoregional relapse-free, and distant metastasis-free survival rates for the 2 groups were compared. Multivariate analysis hazard ratios (HR) with 95% confidence intervals (CI) to identify survival predictors.
Results
Median follow-up was 37 (range, 7–162) months. The 3-year overall, disease-free, locoregional relapse-free, and distant metastasis-free survival rates were 93.8%, 71.6%, 93.5%, and 74.4%, respectively. For those receiving either induction or consolidation chemotherapy, 3-year disease-free survival rates were 82.5% and 67.7%, respectively (P = 0.021), distant metastasis-free rates were 85.4% and 70.8%, respectively (P = 0.024), and both overall and locoregional relapse-free survival rates did not differ significantly. Absence of neural invasion was an independent predictor of disease-free (HR = 0.49, 95% CI 0.25–0.97, P = 0.04) and distant metastasis-free (HR = 0.49, 95% CI 0.25–0.98, P = 0.04) survival. Both ypTN stage III (vs.0-II) and consolidation (vs. induction) chemotherapy were independent predictors of disease relapse (HR = 1.95, 95% CI 1.47–2.58, P < 0.001; HR = 1.68, 95% CI 1.01–2.79, P = 0.046; respectively) and distant metastasis (HR = 2.04, 95% CI 1.51–2.76, P < 0.001; HR = 1.75, 95% CI 1.03–2.99, P = 0.04; respectively).
Conclusions
LARC patients receiving neoadjuvant chemoradiotherapy and total mesorectal excision had better disease-free and distant metastasis-free survival, with induction rather than consolidation neoadjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The standard treatment for locally advanced rectal cancer (LARC) has been neoadjuvant chemoradiotherapy (nCRT) with total mesorectal excision (TME) surgery, often followed by adjuvant chemotherapy (ACT) [1]. Although this trimodal approach has led to a significant reduction in local recurrence rates, distant metastases are the main cause of death, occurring in approximately 30% of these patients [2,3,4]. In addition, treatment with ACT has failed to improve disease-free survival (DFS) and overall survival (OS), and its use is frequently compromised by poor patient tolerance and the need for marked dose reductions [5,6,7].
Consequently, recent treatment strategies for patients with LARC have focused instead on giving additional cycles of chemotherapy prior to TME [2, 8]. The intensification of preoperative treatment with standard dose polychemotherapy administration before surgery, known as total neoadjuvant treatment (TNT), has shown encouraging results [9]. Moreover, the improvement in radiological field could allow to predict pathological response after neoadjuvant treatment; in particular, the assessment of 18F-FDG PET-CT uptake decrease, could guide the choice of administered therapy [10]. In a meta-analysis involving 28 studies, patients with LARC receiving neoadjuvant chemotherapy (NCT) in addition to nCRT had better DFS and OS rates than those receiving nCRT alone [11].
Although NCT is being used increasingly in clinical practice for patients with LARC, the optimal scheduling of NCT and nCRT before TME remains uncertain. The recent CAO/ARO/AIO-12 trial has suggested that the sequencing of NCT and nCRT does seem to affect patient outcomes, demonstrating that nCRT followed by consolidation NCT resulted in the highest pathologic complete response (pCR) rate and clinical complete response (cCR), also reducing grade 3–4 AEs [12]. However, others have noted that it is unknown whether improved pCR rates actually translate into improved survival [13]. The PRODIGE 23 phase III randomized trial [14], has suggested that induction NCT and a shorter course of ACT demonstrated significantly better 3-year DFS (75.7% vs. 68.5%, P = 0.03) and DMFS (78.8% vs. 71.7%, P < 0.02) rates, compared to the group who received the longer course of conventional ACT without NCT. Meanwhile, in the RAPIDO trial [15], compared to the group not receiving NCT, the group receiving consolidation NCT showed a significantly lower risk of disease-related treatment failure (30.4% vs. 23.7%, P = 0.02) and distant metastasis (26.6% vs. 19.8%, P = 0.004). Recently, in the STELLAR trial [16], short-term radiotherapy with preoperative chemotherapy followed by surgery showed noninferiority treatment efficacy compared with standard preoperative nCRT group with regard to disease-free survival (64.5% v 62.3%; P < 0.001 for noninferiority). Additional investigation into the best timing for NCT is still warranted [17].
We were interested in determining the preferable sequence of timing of NCT relative to nCRT in patients with LARC. To explore this issue, we conducted a retrospective study involving patients with LARC who received NCT, nCRT, and TME at our hospital over an 8-year period. We compared the survival rates of patients who were treated with one of two different NCT paradigms, either induction chemotherapy prior to nCRT or nCRT followed by consolidation chemotherapy.
2 Materials and methods
The Institutional Review Board of the Sixth Affiliated Hospital at Sun Yat-sen University approved this retrospective study. The study protocol was approved by the Central Ethics Committee of The Sixth Affiliated Hospital, Sun Yat-sen University (Guangzhou, China) (No. 2020ZSLYEC-289).
2.1 Patient selection
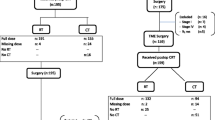
We conducted a retrospective study of consecutive patients with biopsy-proven, locally advanced, non-metastatic rectal cancer who had been treated with nCRT followed by TME at our center between January 2010 and December 2018 (Fig. 1). Of the 1,958 patients who were initially identified, 426 (21.8%) met the following inclusion criteria: (a) had clinical stage II (T3-4N0) or stage III (T1-4N1-2) rectal cancer, (b) had no distant metastasis, and (c) had undergone NCT prior to TME, either induction chemotherapy before nCRT (IC group) or consolidation chemotherapy after nCRT (CC group). Patients were excluded from the study if they had a histologic type of rectal cancer other than adenocarcinoma, had refused surgery or TME with palliative rather than curative intent, had unfinished neoadjuvant radiotherapy course, received brachytherapy, or had missing data about the chemotherapy regimens received.
2.2 Neoadjuvant and adjuvant treatment
All patients in the study received NCT. Patients in the IC group received induction (before radiotherapy) and concurrent (during radiotherapy) chemotherapy; those in the CC group received concurrent (during radiotherapy) and consolidation (after radiotherapy) chemotherapy. A majority but not all of the patients in the study also received ACT, which was given after TME, and was chosen at the discretion of the multi-disciplinary cancer team at our hospital. All patients in this study received a total of at least 8 preoperative chemotherapy cycles. The chemotherapy regimens used consisted of a fluoropyrimidine-based regimen. Chemotherapy typically involved a fluoropyrimidine-based regimen, consisting of one or more of the following: folinic acid with fluorouracil (de Gramont) and oxaliplatin (FOLFOX); capecitabine with oxaliplatin (CAPOX), or capecitabine (Xeloda) alone. Concurrent chemotherapy regimens always included oral capecitabine.
All patients received intensity-modulated radiotherapy (IMRT), and they were treated 5 days a week with 1 fraction daily. The radiotherapy was provided as follows: a total dose of 50 Gy to the planning target volume (PTV) of the gross tumor volume of the primary rectal tumor (GTVp), given in 25 fractions at 2.0 Gy per fraction; 45 Gy to the PTV of the clinical target volume (CTV), given in 25 fractions at 1.8 Gy per fraction. The CTV was defined as the GTV plus areas considered at significant risk of harboring microscopic disease, including the mesorectum (perirectal fascia), perirectal nodes, presacral region, and internal iliac lymph node region. External iliac nodes were included if the primary tumor invaded adjacent organs (cT4) or if the obturator nodes or external iliac nodes were involved.
2.3 Population characteristics
Disease staging was determined at the time of pretreatment evaluation by magnetic resonance imaging (MRI), computed tomography (CT), and/or endorectal ultrasonography (EUS). All patients were staged according to the 7th edition of the American Joint Commission on Cancer (AJCC) staging system [18].
Baseline characteristics evaluated in this study included the following: age, gender, ECOG grade, clinical TNM stage, distance of distal tumor from anal verge, and tumor differentiation. Clinicopathological characteristics included the following: pathologic stage after nCRT and TME (ypTN stage); pathologic complete response (pCR), defined as the absence of viable adenocarcinoma cells in the TME surgical specimen histopathology (ypT0N0); and other post-TME histopathology results (vascular invasion, neural invasion, surgical margin, and circumferential resection margin).
2.4 Follow-up and outcomes
Follow-up duration was defined as the time from the first day of any treatment to either the date of last examination or the date of death. Patients were routinely assessed at 3-month intervals during the first 3 years of care and at 6-month intervals thereafter. Primary outcomes used in this study included the following: OS, measured as the time from the initiation of treatment to death from any cause; DFS, measured as the time from TME to the first disease relapse at any site; locoregional relapse-free survival (LRFS), measured as the time from TME to the first locoregional (within the pelvis) relapse; and distant metastasis-free survival (DMFS), measured as the time from TME to the first distant (outside the pelvis) relapse.
2.5 Statistical methods
The χ2 test was used to compare the distributions of assorted demographic and clinicopathological characteristics for the IC and CC groups. All survival outcome measures were censored on April 27, 2022. Kaplan–Meier survival curves were used to compare patient survival outcomes between two groups. Statistical differences between curves were calculated using the log-rank test. The multivariate Cox proportional hazards model was utilized to assess potentially relevant factors derived from univariate analysis. The hazard ratios (HR) and 95% confidence intervals (CI) were also calculated. All P values were two-sided, and a P-value less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS (version 24.0; SPSS, Inc, Chicago, IL).
3 Results
3.1 Comparison of NCT paradigms
Of the 426 patients included in this study, 80 (18.8%) were in the IC group and 346 (81.2%) were in the CC group. The median age of all patients was 58.0 (range, 20–87) years (Table 1). There were no statistical differences between the two groups in gender, ECOG grade, clinical N stage, clinical TNM stage, distance of distal tumor from anal verge, or tumor differentiation. A larger proportion of patients in the IC group were young (68.8% vs. 46.5% were 58 years old and younger), whereas a larger proportion of patients in the CC group were older (53.5% vs. 31.3% were 59 years old and older), and these differences were significant (P < 0.001). Additionally, 40.0% of patients in the IC group had clinical stage T4 rectal cancer whereas only 15.3% of those in the CC group had clinical stage T4 disease (P < 0.001).
The proportion of patients receiving more than 4 NCT cycles was significantly higher in the IC group than in the CC group (36.3% vs. 5.5%, P < 0.001) (Table 2). On the other hand, the proportion of patients receiving 5 or more ACT cycles was not significantly different in the IC and CC groups (40.0% vs. 50.0%, P = 0.26). Ultimately, the median total number of chemotherapy cycles received by patients in the 2 groups were the same. The median intervals between radiotherapy and TME (56.5 days vs. 56.0 days) were also similar.
The proportion of patients in the IC and CC groups with pCR were not significantly different (22.5% vs. 18.5%, P = 0.41) (Table 3). The rate of downstaging in the two groups was also not significantly different (51.2% vs. 40.5%, P = 0.08). Conversely, a higher proportion of patients in the IC group than in the CC group ultimately had stage ypT4 stage disease (10.0% vs. 4.1%, P = 0.041). The proportion of patients with positive surgical margins in both groups were low and did not differ significantly (0.0% in the IC group and 0.6% in the CC group, P = 0.50).
3.2 Outcomes
The median follow-up for all patients was 37 (range, 7 to 162) months. The 3-year OS, DFS, LRFS, and DMFS rates for all patients were 93.8%, 71.6%, 93.5%, and 74.4%, respectively. Patients in the IC group, relative to those in the CC group, demonstrated significantly better 3-year DFS (82.5% vs. 67.7%, P = 0.024) and DMFS (85.4% vs. 70.8%, P = 0.021) rates (Fig. 2). Patients in the IC group, relative to those in the CC group, also had higher 3-year OS (98.6% vs. 92.4%, respectively, P = 0.438) and LRFS (97.3% vs. 92.5%, respectively, P = 0.391) rates, but these differences were not significant.
Kaplan–Meier analysis curves of 426 patients with newly diagnosed, biopsy-proven, locally advanced, non-metastatic rectal cancer (LARC), treated with neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME), between January 2010 and December 2018, comparing those whose neoadjuvant chemotherapy (NCT) included induction chemotherapy (IC) and those whose NCT included consolidation chemotherapy (CC): a overall survival, b disease-free survival, c locoregional relapse-free survival, and d distant metastasis-free survival. Patients in the IC group had significantly better disease-free survival (P = 0.02) and distant metastasis-free survival (P = 0.02) than consolidation chemotherapy group patients. Differences between the 2 groups for overall survival rates and locoregional relapse-free survival rates were not significant
Based on univariate analysis, the absence of neural invasion (HR = 0.56, P < 0.001) and pCR (HR = 0.28, P < 0.001) were significant predictors of DFS, whereas ypTN stage III vs. 0-II (HR = 1.97, P < 0.001), ypN1-2 vs. ypN0 stage (HR = 2.21, P < 0.001), ypT4 vs. ypT1-3 stage (HR = 1.60, P < 0.001), NCT paradigm of CC vs. IC (HR = 1.77, P = 0.03), and moderately-poorly vs. well differentiated tumor (HR = 1.41, P = 0.02) were significant predictors of disease relapse (Fig. 3a). Similarly, the absence of neural invasion (HR = 0.55, P < 0.001) and pCR (HR = 0.28, P < 0.001) were significant predictors of DMFS, whereas ypTN stage III vs. 0-II (HR = 2.04, P < 0.001), ypN1-2 vs. ypN0 stage (HR = 2.33, P < 0.001), ypT4 vs. ypT1-3 stage (HR = 1.61, P < 0.001), NCT paradigm of CC vs. IC (HR = 1.85, P = 0.02), moderately-poorly vs. well differentiated tumor (HR = 1.37, P = 0.03), and cN1-2 stage vs. cN0 (HR = 1.34, P = 0.04) were significant predictors of distant metastasis (Fig. 3b).
a Univariate analysis of risk of disease relapse for 426 patients with newly diagnosed, biopsy-proven, locally advanced, non-metastatic rectal cancer (LARC), treated with neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME), between January 2010 and December 2018, by baseline, chemotherapy, and outcome characteristics. Results presented as hazard ratios (HR) with 95% confidence intervals (CI) for 3-year disease-free survival. Abbreviations: ACT, adjuvant chemotherapy; CC, consolidation chemotherapy; IC, induction chemotherapy; NCT, neoadjuvant chemotherapy; ECOG, European Cooperative Oncology Group. b Univariate analysis of risk of distant metastasis for 426 patients with newly diagnosed, biopsy-proven, locally advanced, non-metastatic rectal cancer (LARC), treated with neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME), between January 2010 and December 2018, by baseline, chemotherapy, and outcome characteristics. Results presented as hazard ratios (HR) with 95% confidence intervals (CI) for 3-year distant metastasis-free survival). Abbreviations: ACT, adjuvant chemotherapy; CC, consolidation chemotherapy; IC, induction chemotherapy; NCT, neoadjuvant chemotherapy; ECOG, European Cooperative Oncology Group
Based on multivariate analysis, the absence of neural invasion was the only significant independent predictor of 3-year DFS (HR = 0.50, P = 0.04) (Fig. 4a) and of 3-year DMFS (HR = 0.49, P = 0.04) (Fig. 4b). Conversely, both ypTN stage III vs. 0-II and NCT paradigm of CC vs. IC were the only significant independent predictors of disease relapse (HR = 1.95, P < 0.001; HR = 1.68, P = 0.046; respectively) (Fig. 4a) and of distant metastasis (HR = 2.04, P < 0.001; HR = 1.75, P = 0.04; respectively) (Fig. 4b).
a Multivariate analysis of risk of disease relapse for 426 patients with newly diagnosed, biopsy-proven, locally advanced, non-metastatic rectal cancer (LARC), treated with neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME), between January 2010 and December 2018, by the baseline, chemotherapy, and outcome characteristics that had significant results on univariate analysis. Results presented as hazard ratios (HR) with 95% confidence intervals (CI) for 3-year disease-free survival. Abbreviations: CC, consolidation chemotherapy; IC, induction chemotherapy. b Multivariate analysis of risk of distant metastasis for 426 patients with newly diagnosed, biopsy-proven, locally advanced, non-metastatic rectal cancer (LARC), treated with neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME), between January 2010 and December 2018, by the baseline, chemotherapy, and outcome characteristics that had significant results on univariate analysis. Results presented as hazard ratios (HR) with 95% confidence intervals (CI) for 3-year metastasis-free survival. Abbreviations: CC, consolidation chemotherapy; IC, induction chemotherapy
4 Discussion
In this study, we compared the survival rates of patients with LARC who were treated with one of two different NCT paradigms, either induction chemotherapy prior to nCRT or nCRT followed by consolidation chemotherapy, before undergoing TME. We determined that those who received induction NCT had significantly better 3-year DFS and DMFS rates than those who received consolidation NCT. Whereas they also had better 3-year OS and LRFS rates, these differences were not statistically significant.
Recent studies focusing on the assessment of non-tissue sources of cancer, aim to identify the proper medical treatment before surgery is performed, thus allowing a tailored therapy based on cancer sequencing [19]. Moreover, the recent hospital centralization effect has reduced complications and post-operative morbidity for rectal cancer surgery possibly affecting oncological outcomes, too [20]. Over the decades, improved surgical techniques during TME and the addition of nCRT have dramatically reduced the risk of local recurrence for patients with LARC; however, distant metastases have remained a common area of failure for these patients [2,3,4]. Although ACT has been used to try to address this issue, patient compliance with ACT has historically been suboptimal and published clinical trials have not shown consistent benefit [5,6,7]. More recently, NCT has been employed in the preoperative setting in addition to the chemotherapy provided concurrently with radiotherapy during nCRT, in an effort to eliminate subclinical micrometastases and improve survival in patients with LARC. Relative to ACT, NCT has demonstrated significantly better patient compliance, ranging from 81.9% to 100%, and a superior toxicity profile [11, 21,22,23,24].
The FOWARC study is a multicenter, randomized, phase III study conducted at 15 hospitals in China [25]. The study aimed to evaluate superiority of the mFOLFOX-based treatment regimens versus fluorouracil-radiotherapy, in which patients with stage II/III rectal cancer were randomly assigned (1:1:1) to fluorouracil plus radiotherapy followed by surgery and ACT, the same treatment plus mFOLFOX6, or four to six cycles of mFOLFOX6 followed by surgery and ACT. The 3-year DFS was 72.9%, 77.2%, and 73.5% (P = 0.709), and the 3-year overall survival rate was 91.3%, 89.1%, and 90.7% (P = 0.971), respectively. In our study, patients in the IC group demonstrated slightly higher 3-year DFS (82.5%) and OS (98.6%) rates, compared with the fluorouracil plus radiotherapy followed by surgery and ACT group in their research. There is now a growing body of literature showing that the addition of NCT to nCRT and TME in patients with LARC may result in lower metastasis rates and better survival. The addition of NCT has been supported by a systematic review of 10 studies involving 648 patients with LARC who received induction NCT, nCRT, and surgical resection [24]. This review showed excellent results, including 5-year OS and DFS rates of 74.4% and 65.4%, respectively, as well as weighted mean local recurrence and distal failure rates of 3.5% and 20.6%, respectively. The results in our patients were consistent with these, with 3-year OS, DFS, LRFS, and DMFS rates for all patients of 93.8%, 71.6%, 93.5%, and 74.4%, respectively.
Some recent reports have begun to shed light on which NCT paradigm and how many cycles of NCT may be optimal. In the CAO/ARO/AIO-12 study, 306 patients with LARC were given either induction NCT before nCRT or consolidation NCT after nCRT [12]. They reported pCR in 17% of patients receiving induction and 25% of patients receiving consolidation NCT. However, the median interval between radiotherapy and TME was 45 days in their induction group and 90 days in their consolidation group [26]. We wonder if this difference in time intervals may have biased these pCR results, given that tumor regression after radiotherapy is time dependent. In our study, we found that the pCR rate for the patients receiving induction and consolidation did not differ significantly (22.5% vs. 18.5%, P = 0.41), and this was not biased by the median time interval between radiotherapy and TME, which was 56.5 days in the IC group and 56.0 days in the CC group. Also, even if pCR rates are better with consolidation than induction NCT, it is not clear whether this would translate into better metastasis-free or overall survival [13].
The OPRA trial involved patients with LARC who received nCRT and were randomized to 4 months of FOLFOX or CAPEOX before (Induction) or after (Consolidation) group [27]. Patients were re-staged 8 to 12 weeks after completing these treatments. Patients with complete or near-complete clinical response were offered a watch and wait strategy, while those with an incomplete response had TME. The proportion of patients who were offered watch and wait was significantly higher in the consolidation group than the induction group (58% vs. 43%, P = 0.01). The 3-year DFS and DMFS rates in the induction group (78% and 81%, respectively) and in the consolidation group (77% and 83%, respectively) were similar. These differ from the results in our study, in which the 3-year DFS and DMFS rates for patients who received induction NCT (82.5% and 85.4%, respectively) were significantly higher than those for patients who received consolidation NCT (67.7% and 70.8%, respectively).
One possible reason that our DFS and DMFS results differed from those in the OPRA trial is that a higher proportion of patients in our IC group than in our CC group received more than 4 cycles of NCT. In fact, this finding could be interpreted to suggest that patient compliance with NCT was higher in the IC group than the CC group in our study. This same concept about NCT compliance was used by the authors of the CAO/ARO/AIO-12 study, and they reported that compared to patients receiving consolidation NCT, a higher proportion of those receiving induction NCT received full doses of all recommended NCT cycles [12]. As the retrospective nature of our study, the actual compliance of these two paradigms is difficult to evaluate. We speculate that the inferior compliance with consolidation NCT in our study may have had an adverse effect on the DFS and DMFS rates of patients in the CC group. It is also possible that earlier exposure to systemic chemotherapy may have reduced the incidence of distant failure in the IC group. However, whether earlier administration of and good compliance with NCT translates directly into better long-term survival is still unknown.
This study has some limitations. First, aside from potential biases because it is a retrospective study from a single center, there were other potential sources of bias. For example, some imaging-relevant pretreatment characteristics we concerned were missed due to unavailable or poor-quality MRI scans, such as mesorectal fascia (MRF), extramural venous invasion (EMVI) and lateral lymph node (ILN). These prognostic factors can influence choices regarding NCT and may have led to selection bias. As another example, a significantly higher proportion of patients in the IC group, compared to those in the CC group, were younger and had clinical stage T4 rectal cancer. Although these differences could have biased our results, neither of these characteristics turned out to be significant prognostic factors in multivariate analysis. Second, there was considerable heterogeneity in the chemotherapy regimens (including ACT) and number of cycles of chemotherapy administered to patients in our study. This was in part because of the paucity of conclusive data concerning the use of NCT in LARC. Despite this, the regimens that we used for our patients were consistent with the National Comprehensive Cancer Network (NCCN) guidelines [1]. These differences could potentially have impacted the oncological outcomes in our study. However, it is noteworthy that the median total number of chemotherapy (combined NCT and ACT) cycles received was 8 in both of our study groups, and the heterogeneity of the treatment regimens was unavoidable given the retrospective nature of the study. Finally, although we did find better DFS and DMFS rates in the IC group, this cohort was relatively small. Because of this and the retrospective nature of our study, our findings should be interpreted with caution.
5 Conclusion
Patients receiving nCRT and TME for LARC had better disease-free and metastasis-free survival with the addition of induction NCT rather than consolidation NCT to their treatment regimen.
Data availability
The datasets used for this current study are available from the corresponding author upon reasonable request.
Abbreviations
- LARC:
-
Locally advanced rectal cancer
- nCRT:
-
Neoadjuvant chemoradiotherapy
- TME:
-
Total mesorectal excision
- ACT:
-
Adjuvant chemotherapy
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- LRFS:
-
Locoregional relapse-free survival
- DMFS:
-
Distant metastasis-free survival
- pCR:
-
Pathologic complete response
- FOLFOX:
-
Folinic acid, fluorouracil, and oxaliplatin
- CAPOX:
-
Capecitabine and oxaliplatin
- Xeloda:
-
Capecitabine
- IMRT:
-
Intensity-modulated radiotherapy
- GTV:
-
The gross tumor volume
- CTV:
-
The clinical target volume
- AJCC:
-
The American joint commission on cancer
- NCCN:
-
The national comprehensive cancer network
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
References
Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):874–901.
Gollins S, Sebag-Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol. 2016;28(2):146–51.
van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223–9.
Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90.
Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356–62.
Franke AJ, Parekh H, Starr JS, et al. Total neoadjuvant therapy: a shifting paradigm in locally advanced rectal cancer management. Clin Colorectal Can. 2018;17(1):1–12.
Guida AM, Sensi B, Formica V, et al. Total neoadjuvant therapy for the treatment of locally advanced rectal cancer: a systematic minireview. Biol Direct. 2022;17(1):16.
Caruso R, Vicente E, Quijano Y, et al. Role of 18F-PET-CT to predict pathological response after neoadjuvant treatment of rectal cancer. Discov Oncol. 2021;12(1):16.
Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–8.
Fokas E, Allgäuer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37(34):3212–22.
Petrelli F, Borgonovo K, Cabiddu M, et al. Pathologic complete response and disease-free survival are not surrogate endpoints for 5-year survival in rectal cancer: an analysis of 22 randomized trials. J Gastrointest Oncol. 2017;8(1):39–48.
Thierry Conroy NL, Pierre-Luc Etienne, Emmanuel Rio, Eric FRANCOIS, Nathalie mesgouez-nebout, total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020; 38: 2020
van der Valk MJM, Marijnen CAM, van Etten B, et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—results of the international randomized RAPIDO-trial. Radiother Oncol. 2020;147:75–83.
Jin J, Tang Y, Hu C, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. 2022;40(15):1681.
Abdalla A, Aref A. Upfront chemotherapy followed by chemoradiation remains the sequence of choice for total neoadjuvant chemotherapy for locally advanced rectal cancer. J Clin Oncol. 2019;37(36):3561–2.
Edge SB, Compton CC. The American joint committee on cancer the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Amelio I, Bertolo R, Bove P, et al. Liquid biopsies and cancer omics. Cell Death Discov. 2020;6(1):131.
Siragusa L, Sensi B, Vinci D, et al. Volume-outcome relationship in rectal cancer surgery. Discov Oncol. 2021;12(1):11.
Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: grupo cancer de recto 3 study. J Clin Oncol. 2010;28(5):859–65.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071.
Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):1474–5488.
Zaborowski AA-O, Stakelum AA-OX, Winter DC. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. Br J Surg. 2019;106(8):979–87.
Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37(34):3223–33.
Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean J-P, Partensky C, Souquet JC, Adeleine P, Gerard J-P, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90–01 randomized trial. J Clin Oncol. 1999. https://doi.org/10.1200/JCO.1999.17.8.2396.
Julio Garcia-Aguilar SP, Kim JK, Yuval JB, Thompson H, Verheij F, Lee M, Saltz LB. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38:2020 (suppl; abstr 4008).
Funding
This study was supported by Natural Science Foundation of China (NO. 82171163).
Author information
Authors and Affiliations
Contributions
ZJ and SJC designed the study; HF and CM performed the contouring, treatment planning, and statistical analysis; LYP, and ZJ reviewed the data; all authors discussed the data; HF and CM drafted the manuscript; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by by the Central Ethics Committee of The Sixth Affiliated Hospital, Sun Yat-sen University (Guangzhou, China) (No. 2020ZSLYEC-289). Written informed consent was obtained from individual or guardian participants.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, F., Chen, M., Liu, Yp. et al. Timing of additional neoadjuvant chemotherapy in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy and total mesorectal excision. Discov Onc 13, 111 (2022). https://doi.org/10.1007/s12672-022-00572-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-022-00572-4