Abstract
Background
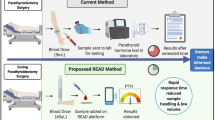
Intraoperative parathyroid hormone (ioPTH) is used during minimally invasive parathyroidectomy (MIP) to predict the success of surgery and should be accurate with a short turnaround time.
Material and method
We developed an ioPTH point-of-care (POC) assay on Philips handheld magnotech system. Magnotech technology is based on magnetically controlled movement of superparamagnetic nanoparticles in stationary sample fluid. During first phase, intact-PTH is captured by magnetic particles coated with anti-N-terminal-PTH antibodies. Subsequently, magnetic particles are collected by magnetic forces at sensor surface coated with anti-C-terminal-PTH antibodies. Unbound/nonspecifically bound particles are pulled away from detection surface, using a second magnetic force. Amount of specifically bound particles is measured using a surface-sensitive optical imaging technique.
Results
ioPTH test could be performed with a turnaround time of less than 10 min and could detect low intact-PTH concentrations (picomolar). Integrated cartridge contains a blood separation filter and dry reagents for the assay.
Conclusion
The next magnotech ioPTH assay will be the only POC test able to give accurate results in less than 10 min, using 25 μL of whole blood. Thanks to the ease-of-use, magnotech ioPTH could be performed in the operating theater by any member of surgical staff.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) is caused by a single-gland disease in more than 80% of patients, for whom unilateral cervical exploration would be beneficial compared with a four-gland exploration [1]. The improvement in imaging with 99mTc-labeled sestamibi scintigraphy and ultrasonography of the neck to localize a single adenoma preoperatively, has made minimally invasive parathyroidectomy (MIP) the preferred approach to PHPT for most endocrine surgeons [2, 3]. Upto 92% of patients with positive localization studies undergo MIP [4]. MIP is often coupled with intraoperative parathyroid hormone (ioPTH) monitoring during surgery to confirm the completeness of resection of the hyperfunctioning parathyroid gland and to reveal cases of multiple-gland parathyroid disease not recognized on imaging [5, 6]. Such approaches can reduce the costs of medical care by decreasing the duration of the surgery and lengths of stay, changing from an inpatient to an outpatient procedure [7]. Patient satisfaction is also greater with respect to cosmetic result and postoperative pain.
Intact-PTH consists of a single polypeptide chain, containing 84 amino acids with amino and carboxy terminals, which is produced only in the parathyroid glands and secreted into the bloodstream. The circulating (1–84) PTH molecule has a half-life of only 5 min. Blood concentrations of intact-PTH rapidly decrease after removal of hypersecreting parathyroid gland. Typically, PTH concentrations are measured at baseline, before exploration, and then at 5–10 min post-excision, with a 50% decrease in PTH values from the highest baseline observed if all hypersecreting tissue has been removed [8]. Since the mid-1990s, the development of rapid ioPTH assays, improving the turnaround time of the result, allowed for its practical use in the surgical management of PHPT [9, 10]. Rapid ioPTH assays have incubation times ranging from 10 to 30 min, giving turnaround times of up to 1 h. Most of the laboratories performing rapid PTH testing are carrying out testing in the central laboratory on an automated analyzer [11]. An on-site approach, i.e., operating theater testing requires additional instrument and operator training and a dedicated laboratory technician.
Royal Philips Electronics has developed a handheld immunoassay system called magnotech for the point-of-care (POC) testing that can measure picomolar concentrations of analytes in 10 min or less [12–14]. We worked on the development of an ioPTH assay on the magnotech system with a turnaround time of less than 10 min [15, 16]. Such an assay would have the potential to be used in the operating theater. We present here the preliminary results obtained internally with an in-house magnotech ioPTH assay.
Materials and methods
Magnotech technology
The diagnostic system is based on Philips' proprietary magnotech immunoassay technology (http://www.philips.com/magnotech) [13, 14]. The system consists of a handheld analyzer instrument and a disposable self-contained cartridge. Magnetic nanoparticles that have been functionalized with antibodies are used to capture and to detect the analyte molecules. Magnetic forces are used to transport the nanoparticles to the sensor surface, which is also coated with antibodies where they can bind. By collecting all magnetic particles near the sensor surface, a significant increase in analyte concentration is achieved, which speeds up the binding kinetics, shortening the assay time. All assay reagents are stored inside the cartridge in a dry form; therefore, once the sample liquid has been added to the cartridge, no further operator interaction is required. The short measurement time, in combination with the high ease-of-use, makes the test very suitable for ioPTH testing use, enabling the surgical staff to perform the measurement during the operation without the need for a laboratory technician. The magnotech system uses the principle of frustrated total internal reflection (FTIR) to detect the presence of any magnetic nanoparticles bound to the sensor surface. The FTIR principle is illustrated in Fig. 1. Light is projected on the surface of the cartridge at an angle that is slightly shallower than the critical angle, causing a reflection that is imaged on a camera sensor. In the absence of any magnetic particles, the beam of light is reflected at full intensity, resulting in a bright image. When nanoparticles did bind on the sensor surface, some of the light is reflected and scattered by the nanoparticles, resulting in a decreased intensity of the reflected light. This decrease in signal is proportional to the number of particles bound, and hence, to the analyte concentration.
Magnotech ioPTH assay
We have developed an immunoassay on magnotech system for the in vitro quantitative determination of intact PTH in human blood. Magnotech ioPTH assay uses a sandwich test principle in which a monoclonal antibody reacts with the N-terminal fragment (1–34) of PTH, and a second antibody reacts with the C-terminal fragment (39–84) of PTH. In the first step of the assay, the PTH molecules are captured on magnetic nanoparticles coated with anti-N-terminal PTH antibodies (Fig. 2a). Since the combined surface area of the magnetic particles is large, this capture phase goes very quickly. In the next step of the assay, all magnetic particles are attracted by a magnetic force directed towards the sensor surface covered with an anti-C terminal PTH antibody (Fig. 2b). The magnetic particles that captured a PTH molecule have a chance to bind to the antibodies on the detection spot in a sandwich assay format. However, many particles that did not capture any PTH were also attracted, but these cannot bind. The unbound labels are removed by a magnetic “washing” step that consists of a magnetic force directed away from the sensor surface (Fig. 2c). After the magnetic washing step, only the specifically bound labels remain at the sensor surface and the unbound beads have been moved into the bulk of the liquid. The number of bound labels is finally quantified, using a surface-sensitive technique based on the FTIR principle. In this assay format, no other liquids are present besides the sample liquid, making it a truly homogeneous assay. All assay reagents are stored inside the cartridge in a dry form, so no additional sample pretreatment steps are required and the sample can be added directly to the cartridge.
Magnotech assay principle. During the first step of the assay, the PTH molecules are captured on magnetic nanoparticles (a). Next, the magnetic particles are attracted towards the sensor surface, where the labels that captured a target can bind in a sandwich format (b). Finally, the unbound magnetic particles are removed by a magnetic force directed away from the sensor surface (c) after which the number of bound labels is quantified. (Reprinted with permission of Philips)
Functionalization of carboxylated-magnetic nanoparticles
Magnetic nanoparticles were coated with anti-N-terminal PTH antibodies, using EDC (N-Ethyl-N′-(3-dimethylaminopropyl) carbodiimide) chemistry, as described by Dittmer et.al. [12]. Tracer anti-N-terminal PTH antibody was added to 10 mg/mL of superparamagnetic particles functionalized with carboxylic acid groups (500 nm in diameter) to a final concentration of 20 μg antibody/mg particles in 50 mM of MES (2-N-morpholinoethanesulfonic acid; pH 6.2) and incubated for 30 min on a roller-mixer at room temperature (RT). Next, the antibodies were coupled to the carboxyl groups of the particles with 2 mg/mL EDC (Sigma–Aldrich Corp.) for 30 min on a roller-mixer at RT. Finally, the reaction was inactivated with 100 mM Tris (pH 8), and the functionalized particles were transferred to a storage buffer.
Production of integrated PTH cartridges
The integrated cartridge consisted of three components, e.g., fluidic part, optical part, and a double-sided biocompatible adhesive tape.
The fluidic part is the plastic top part of the cartridge and was injection-molded in-house (Philips, Eindhoven, The Netherlands). Functionalized particles (200 nL) were deposited into a cavity in the top plastic part of the cartridge, using the Nanodrop NS-2Stage (Innovadyne Technologies, Inc. Carnforth, UK). The particles were dried at 37°C for 1 h, and subsequently, sealed with silica bags and stored at 4°C.
The optical part is the plastic bottom part of the integrated cartridge, comprising the detection surface and was formed by the injection molding of high binding microtiter plate substrate material. The detection spot was functionalized with anti-C-terminal PTH antibody (2 nL; 175 μg/mL), using an inkjet printer (sciFLEXARRAYER S3, Scienion AG), as described by Dittmer et.al. [12]. Functionalized optical parts were sealed with silica bags and stored at 4°C.
Finally, the cartridge was assembled by connecting the fluidic part and the optical part with double-sided tape that was laser-cut to form a sample inlet, microfluidic channel, a reaction chamber, and a vent. With a 100-μm tape and with a 180-μm tape, a reaction chamber of 0.4 μL and a reaction chamber of 0.5 μL were created, respectively. The integrated PTH cartridges were sealed with silica bags and stored at 4°C.
EDTA-plasma samples
EDTA-plasma unit used for the PTH-standard curve was purchased from ProMedDx (MA, USA).
To prove the linear correlation between PTH concentrations covering the clinical relevant range (>70 pg/mL), and the signals obtained with the magnotech ioPTH assay, we used 20 EDTA-plasma samples with PTH levels measured with a commercially available reference PTH assay (Intact PTH, Roche Modular Analytics E170, Roche Diagnostics, Germany). These samples from anonymous patients screened for parathyroid disorders, were left-over samples acquired from Labor Limbach (Heidelberg, Germany).
To demonstrate that magnotech ioPTH assay is able to detect a 50% decrease of PTH levels after resection of the hyperfunctioning parathyroid tissue, blood samples from two patients, undergoing parathyroid adenoma surgery, were collected and tested on the magnotech system. The EDTA-blood samples taken before surgery, just before excision, and several time points after resection, were centrifuged and, as a reference, PTH levels were measured in a microtiter plate-based assay (STAT-intraoperative intact-PTH assay, Future Diagnostics, Wijchen, The Netherlands) [17].
ioPTH assay protocol
Either native PTH plasma samples or PTH-spiked plasma were measured. For the latter, intact-PTH (Bachem, Germany) was spiked to PTH-free EDTA plasma. PTH levels were measured by injecting 10 μL of sample into the inlet of the cartridge. After complete filling of the reaction chamber, the actuation protocol was started. The total assay time was 8 min. Unless otherwise stated, all data points are from assays that were performed in duplicates.
Results
PTH-dose response curve and precision
Using cartridges that were functionalized with anti-C-terminal PTH antibodies on the detection spot, as well as anti-N-terminal PTH antibodies on the magnetic particles, and the magnotech technology, a PTH-dose response curve was generated in EDTA-plasma. Various concentrations of PTH were spiked in PTH-free EDTA plasma, and PTH levels were measured with an 8-min assay protocol, using 0.5 μL of final sample volume (reaction chamber) (Fig. 3). PTH-free EDTA plasma was measured in 12-fold and each PTH-spiked plasma sample was measured in fivefold. At the higher end of the PTH-dose response curve, a plateau in signal (80 arbitrary units) was obtained for PTH concentrations ≥2,500 pg/mL. A linear correlation(r = 0.997) between concentrations and signals was found for PTH concentrations, ranking from 0 to 1,500 pg/mL. Furthermore, PTH levels down to normal values (<70 pg/mL) could significantly be measured. These findings implicate that samples from patients with parathyroid adenoma who have high PTH concentrations falling down to normal values during surgery, could be accurately tested with the magnotech ioPTH assay. The magnotech ioPTH assay is aimed for a 20% CV (coefficient of variation) at the lower limit of PTH normal values (10 pg/mL). The results obtained in this study suggest that this objective can be reached with a turnaround time of less than 10 min. In addition, the CV in the linear range was calculated (n = 28) in EDTA-plasma with a PTH concentration of 500 pg/mL. The CV was typically less than 10% (data not shown).
PTH dose–response curve measured with integrated PTH cartridges. Using cartridges that contained a detection spot with anti-C-terminal PTH antibodies and beads coated with anti-N-terminal PTH antibodies, PTH-free EDTA plasma was measured in 12-fold and the PTH (1–84)-spiked plasma samples were measured in fivefold. PTH levels were measured in a detection volume of 0.5 μL with an 8-min assay
Comparative study on native samples
The linear correlation between PTH levels and signals obtained with the magnotech ioPTH assay was further demonstrated using 20 EDTA-plasma samples with a range of native PTH concentration from 68 to 1,958 pg/mL measured on a reference system. These samples were measured with an 8-min protocol, using 0.4 μL of final sample volume in the integrated PTH cartridge (Fig. 4). We found a linear correlation (r = 0.93) between PTH values and signals obtained with magnotech ioPTH assay. Thus, the magnotech ioPTH assay could be suitable for evaluation of the 50% drop of PTH levels in plasma samples collected during parathyroid adenoma surgery.
Correlation on a limited number of native PTH samples. Native PTH EDTA-plasma samples (n = 20) with PTH concentrations measured on the Roche Modular system were evaluated on the Philips biosensor. The signal (arbitrary units) induced by these samples were measured in cartridges that contained a detection spot with anti-C-terminal PTH antibodies and beads coated with anti-N-terminal PTH antibodies, using 0.4 μL detection volume and an 8-min assay protocol
Fifty-percent drop of PTH after resection of parathyroid gland
During two surgeries of parathyroid adenomas, EDTA-plasma samples were collected before surgery (M1), just before resection of the gland (M2), 5 min (M3), and 10 min (M4) after resection. For patient #2, an additional sample at 20 min after resection (M5) was taken. PTH levels in these samples were measured with the STAT-intraoperative intact-PTH assay (Future Diagnostics) on site (Fig. 5a and c). In both patients, the PTH levels before surgery were dramatically higher than normal PTH levels (patient #1: 270 pg/mL; patient #2: 900 pg/mL). After a freeze-thaw step, PTH levels in these samples were measured with an 8-min protocol, using 0.4 μL of final sample volume (reaction chamber) with the magnotech ioPTH assay (Fig. 5b and d). For both patients, the PTH level in sample M2 was used to normalize the signal obtained with the integrated PTH cartridge to 100% and, next, a clear 50% reduction of PTH concentration was found in the sample that was collected 10 min after resection (sample M4).
Fifty-percent drop of PTH levels 10 min after resection of the parathyroid adenoma. Various EDTA-plasma samples were collected during two surgeries of parathyroid adenomas; samples before surgery (M1), just before resection (M2), 5 min (M3), 10 min (M4), and 20 min (M5) after resection. These samples were measured with the STAT-intraoperative intact-PTH assay on site (a and c) and, after a freeze-thaw step, the signal (arbitrary units) induced by these samples were measured in cartridges that contained a detection spot with anti-C-terminal PTH antibodies and anti-N-terminal PTH antibody-coated beads, using 0.4 μL detection volume and an 8-min assay protocol. The PTH levels in sample M2 were used to normalize the signal obtained with the magnotech ioPTH assay to 100% (b and d)
Discussion
In this paper, we report a number of experiments that we performed to illustrate the potential of the ioPTH assay on the Philips handheld magnotech system. The work was performed in a research setting that imposed a number of limitations. The disposable cartridges were assembled in a highly manual process, which likely introduced some additional variations to the test results. Furthermore, the experiments were performed on a very limited number of native samples, which does not allow for a detailed statistical analysis. Finally, the measurements with the magnotech ioPTH assay presented in this study, were performed with plasma as a sample matrix, where whole blood will be required in the final application for optimal ease-of-use; the next step will be to investigate how a plasma separation membrane can be added to the disposable cartridge, which would enable a measurement using whole blood samples. This will remove the centrifugation step currently in use to obtain the plasma, replacing it with a step which is integrated in the cartridge and significantly faster (<1 min). Despite these limitations, the preliminary results we report provide an important step towards a clinically usable POC ioPTH assay on magnotech system.
The PTH-dose response curve illustrates a linear correlation between concentrations of PTH up to 1,500 pg/mL and signals obtained with the magnotech ioPTH assay. As a consequence, a 50% reduction of signal, expected after resection of the hyperfunctioning parathyroid gland, could be determined with the magnotech ioPTH assay. The experiments were performed, using integrated cartridges that contain all reagents in a dry form, which proves that it is possible to achieve good sensitivity in very short assay times. The error bars were already quite small, and these are expected to further improve, once the disposable cartridges are manufactured in a more automated fashion.
The comparative study shows that the results obtained with the magnotech ioPTH assay correlate well with the reference system. The observed scatter is likely due to the combined effect of the characteristics of the individual samples, the relatively low number of repeats, and the aforementioned highly manual manufacturing method of the cartridge. Despite all these, a very good correlation (r = 0.93) was already obtained in this early phase, which should be considered as very encouraging.
The most convincing argument is formed by the measurements that were carried out on samples from two patients during surgery. The drop in PTH levels after removal of the diseased gland is clearly visible, and could be used to provide a surgeon with the immediate feedback that the operation has been successful. The good correlation between the magnotech ioPTH assay and the STAT assay (Future Diagnostics) used as a reference method, again, looks promising.
Based on our preliminary results with an in-house magnotech ioPTH assay, we are confident that the next commercially available magnotech ioPTH assay could meet the requirements for ioPTH testing in the operating theater. Further studies are necessary, including analytical, clinical, and health economics studies, to validate the magnotech ioPTH assay as a reliable and cost-effective solution to measure intraoperative PTH in patients, undergoing parathyroidectomy.
Conclusion
We have developed a POC assay for ioPTH testing based on Philips' magnotech handheld system. The PTH-dose response curve shows that the clinically relevant concentrations of PTH can be accurately detected with a turnaround time of less than 10 min. PTH measurements with the magnotech ioPTH system on native plasma samples are well correlated with the values obtained on a reference system. Finally, and most relevantly, measurements performed on samples from patients undergoing parathyroidectomy show that a 50% drop in PTH concentration could be clearly detected after the gland was removed. These results illustrate the potential of the next magnotech ioPTH assay to be a viable and easy-to-use solution to perform ioPTH measurements in the operating theater.
Abbreviations
- ioPTH:
-
Intra-operative PTH
- PTH:
-
Parathyroid hormone
- POC:
-
Point-of-care
- MIP:
-
Minimal invasive parathyroidectomy
- PHPT:
-
Primary hyperparathyroidism
- FTIR:
-
Frustrated total internal reflection
- RT:
-
Room temperature
- CV:
-
Coefficient of variation
References
Wells SA, Leight GS, Ross AJ (1980) Primary hyperparathyroidism. Curr Probl Surg 17(8):398–463
Henry JF, Defechereux T, Gramatica L, de Boissezon C (1999) Minimally invasive videoscopic parathyroidectomy by lateral approach. Langenbecks Arch Surg 384(3):298–301
Sackett WR, Barraclough B, Reeve TS, Delbridge LW (2002) Worldwide trends in the surgical treatment of primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Arch Surg 137:1055–1059
Carling T, Udelsman R (2008) Focused approach to parathyroidectomy. World J Surg 32:1512–1517. doi:10.1007/s00268-008-9567-z
Gurnell EM, Thomas SK, McFarlane I, Munday I, Balan KK, Berman L et al (2004) Focused parathyroid surgery with intraoperative parathyroid hormone measurement as a day-case procedure. Br J Surg 91:78–82
Norman J, Chheda H, Farrell C (1998) Minimally invasive parathyroidectomy for primary hyperparathyroidism: decreasing operative time and potential complications while improving cosmetic results. Am Surg 64:391–395
Udelsman R (2002) Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg 235:665–672
Carter AB, Howanitz PJ (2003) Intraoperative testing for parathyroid hormone: a comprehensive review of the use of the assay and the relevant literature. Arch Pathol Lab Med 127(11):1424–1442
Johnson LR, Doherty G, Lairmore T, Moley JF, Brunt LM, Koenig J et al (2001) Evaluation of the performance and clinical impact of a rapid intraoperative parathyroid hormone assay in conjunction with preoperative imaging and concise parathyroidectomy. Clin Chem 47:919–925
Sokoll L, Wians FH, Remaley A (2004) Rapid intraoperative immunoassay of parathyroid hormone and other hormones: a new paradigm for point-of-care testing. Clin Chem 50(7):1126–1135
Hortin GL, Carter AB (2002) Intraoperative parathyroid hormone testing: survey of testing program characteristics. Arch Pathol Lab Med 126:1045–1049
Dittmer WU, Evers TH, Hardeman WM, Huijnen W, Kamps R, de Kievit P, Neijzen JH, Sijbers MJ, Nieuwenhuis JH, Dekkers DW, Hefti MH, Martens MF (2010) Rapid, high sensitivity, point-of-care test for cardiac troponin based on optomagnetic biosensor. Clin Chim Acta 411:868–873
Bruls DM, Evers TH, Kahlman J, van Lankvelt P, Ovsyanko M, Pelssers E, Schleipen J, de Theije F, Verschuren C, van der Wijk T, van Zon J, Dittmer W, Immink A, Nieuwenhuis J, Prins M (2009) Rapid Integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip 9(24):3504–3510
Viegers T, Pelssers E, Lenders E (2008) A microfluidics platform for in vitro testing. Med Device Technol 19(2):23–25
Dittmer WU, de Kievit P, Prins MW, Vissers JL, Mersch ME, Martens MF (2008) Sensitive and rapid immunoassay for parathyroid hormone using magnetic particle labels and magnetic actuation. J Immunol Methods 338:40–46
Jarrige V, Nieuwenhuis J, van Son J, Martens MF, Vissers JL (2010) A fast intra-operative PTH point-of-care assay on the Philips handheld magnotech system. Oral presentation, ESES 4th Biennal congress, Vienna, May 14th
Carneiro DM, Irvin GL 3rd (2002) New point-of-care intraoperative parathyroid hormone assay for intraoperative guidance in parathyroidectomy. World J Surg 26(8):1074–1077
Conflicts of interest
All the authors are employees of Philips or Future Diagnostics, and are involved in the development of the magnotech platform and intraoperative PTH application.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Jarrige, V., Nieuwenhuis, J.H., van Son, J.P.H.F. et al. A fast intraoperative PTH point-of-care assay on the Philips handheld magnotech system. Langenbecks Arch Surg 396, 337–343 (2011). https://doi.org/10.1007/s00423-010-0733-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-010-0733-z