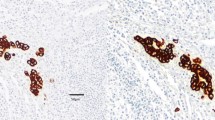
Abstract
Background and aims
Isolated tumor cells (ITCs) in cancer patients are retrieved mostly using immunohistochemistry with antibodies directed against antiepithelial antigens (for example Ber-EP4), which are supposed not to be present in metastatic-free tissue. To date, there has been ongoing controversy whether those cells have biologic significance and are linked with tumor progression and impaired patient’s prognosis. Therefore, the aim of this study was to further characterize Ber-EP4-positive cells in various tissues, with special emphasis on their tumorigenic origin.
Materials and methods
The frequency and prognostic impact of ITCs in lymph nodes displayed by means of monoclonal antibody Ber-EP4 were evaluated in retrospective (n = 292) and prospective (n = 100) collectives of various gastrointestinal carcinomas free of metastatic disease in conventional histopathology (pN0). Furthermore, the frequency of ITCs in the peritoneal cavity and bone marrow was analyzed in case of absence of overt distant metastasis (pM0) in the prospective collective. Ber-EP4-immunoreactive cells were further characterized for tumorigenic origin using morphological criteria and immunohistochemical double staining for Ber-EP4 and p53.
Results
Ber-EP4-positive cells could be revealed in lymph nodes in 44.3% of pN0-gastrointestinal carcinomas, in the peritoneal cavity in 19%, and in the bone marrow in 10%. In lymph nodes, BerEP4-immunoreactive cells exhibited a metastatic-atypical morphology in 59%; however, it was always typical for true tumor cells in the peritoneal cavity or bone marrow. The cumulative 5-year survival rate was adversely affected by Ber-EP4-immunoreactive cells in uni- and multivariate analysis, irrespective of the underlying cell morphology (68% for Ber-EP4 negative, 41% for Ber-EP4 positive with atypical and typical morphology each). In the case of a p53-positive primary tumor, 70% of the corresponding ITCs also overexpressed p53, while the remainder was deemed p53 negative (p = 0.002).
Conclusion
ITCs detected by the antiepithelial antibody Ber-EP4 are present in a substantial proportion of apparently tumor-free lymph nodes. These cells impair patients’ prognoses, irrespective of the underlying cell morphology. As approximately one third of Ber-EP4-positive cells in p53-positive primary tumors do not overexpress p53; their true tumorigenic origin needs to be further investigated.





Similar content being viewed by others
References
Sobin LH, Wittekind CH (2002) TNM classification of malignant tumors, 6th edn. Wiley-Liss, New York
Dobashi K, Sugio K, Osaki T, Oka T, Yasumoto K (1997) Micrometastatic p53-positive cells in the lymph nodes of non-small cell lung cancer: prognostic relevance. J Thorac Cardiovasc Surg 114:339–346
Gu CD, Osaki T, Oyama T, Inoue M, Kodate M, Dobashi K, Oka T, Yasumoto K (2002) Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer. Ann Surg 235:133–139
Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE, Pantel K (1997) Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with complete resected esophageal cancer. N Engl J Med 337:1188–1194
Inoue S, Nakao A, Kasai Y, Harada A, Nonami T, Takagi H (1995) Detection of hepatic micrometastasis in pancreatic adenocarcinoma patients by two-stage polymerase chain reaction/restriction fragment length polymorphism analysis. Jpn J Cancer Res 86:626–630
Hosch SB, Knoefel WT, Metz S, Stoecklein N, Neindorf A, Broelsch CE, Izbicki J (1997) Early lymphatic tumor cell dissemination in pancreatic cancer: frequency and prognostic significance. Pancreas 15:154–159
Milsmann C, Füzesi L, Werner C, Becker H, Horstmann O (2005) Okkulte lymphatische Tumorzelldisseminierung beim Pankreaskarzinom. Der Chirurg 76:1064–1072
Horstmann O, Fuzesi L, Markus PM, Werner C, Becker H (2004) Significance of isolated tumor cells in lymph nodes among gastric cancer patients. J Cancer Res Clin Oncology 130(12):733–740
Adell G, Boeryd B, Franlund B, Sjördahl R, Hakansson L (1996) Occurrence and prognostic importance of micrometastases in regional lymph nodes in Dukes’ B colorectal carcinoma: an immunohistochemical study. Eur J Cancer 162:637–642
Broll R, Schauer V, Schimmelpenning H, Strik M, Woltmann A, Best R, Bruch HP, Duchrow M (1997) Prognostic relevance of occult tumor cells in lymph nodes of colorectal carcinomas: An immunohistochemical study. Dis Colon Rectum 40:1465–1471
Domagala W, Bedner E, Chosia M (1991) Keratin-positive reticulum cells in fine needle aspirates and touch imprints of hyperplastic lymph nodes. Acta Cytol 36:241–251
Traweek ST, Liu J, Battifora H (1993) Keratin gene expression in non-epithelial tissues. Am J Pathol 142:1111–1118
Pantel K, Schlimok G, Angstwurm M, Weckermann D, Schmaus W, Gath H, Passlick B, Izbicki JR, Riethmüller G (1994) Methodological analysis of immunocytochemical screening for disseminated epithelial tumor cells in bone marrow. J Haematother 3:165–173
Jung R, Petersen K, Krüger W, Wolf M, Wagener C, Zander A, Neumaier M (1999) Detection of micrometastasis by cytokeratin 29 RT-PCR is limited due to stable background transcription in granulocytes. Br J Cancer 81:870–873
Passlick B, Izbicki JR, Kubuschok B, Nathrath W, Thetter O, Pichelmaier U, Schweiberer L, Riethmüller G, Pantel K (1994) Immunohistochemical assessment of individual tumour cells in lymph nodes of patients with non-small cell lung cancer. J Clin Oncol 12:1827–1832
Latza U, Niedobitek G, Schwarting R, Nekarda H, Stein H (1990) Ber-Ep4: new monoclonal antibody which distinguishes epithelia from mesothelia. J Clin Pathol 43:213–219
Momburg F, Moldenhauer G, Hämmerling GJ, Möller P (1987) Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissue. Cancer Res 47:2883–2891
Motherby H, Friedrichs N, Kube M, Nadjari B, Knops K, Donner A, Baschiera B, Dalquen P, Böcking A (1999) Immunocytochemistry and DNA-image cytometry in diagnostic effusion cytology. Anal Cell Pathol 19:7–20
Baily ME, Brown RW, Mody DR, Cagle P, Ramzy I (1996) Ber-EP4 for differentiating adenocarcinoma from reactive and neoplastic mesothelial cells in serous effusions. Acta Cytol 40:1212–1216
DeAngelis M, Buley ID, Heryet A, Gray W (1992) Immunocytochemical staining of serous effusions with monoclonal antibody Ber-EP4. Cytopathology 3:111–117
Diaz-Arias AA, Loy TS, Bickel JT, Chapman RK (1993) Utility of Ber-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn Cytopathol 9:516–521
Djemek A, Brockstedt U, Hjerpe A (1997) Optimisation of a battery using nine immunohistochemical variables for distinguishing between epithelial mesothelioma and adenocarcinoma. APMIS 105:889–894
Gaffey MJ, Mills SE, Swanson PE, Zarbo RJ, Shah AR, Wick MR (1992) Immunoreactivity for Ber-EP4 in adenocarcinomas, adenoid tumors, and malignant mesotheliomas. Am J Surg Pathol 16:593–599
Grove A, Paulsen SM, Gregersen M (1994) The value of immunohistochemistry of pleural biopsy specimens in the differential diagnosis between malignant mesothelioma and metastatic carcinoma. Pathol Res Pract 190:1044–1055
Jensen ML, Johansen P (1996) Immunocytochemical staining of smears and corresponding cell blocks from serous effusions: a follow-up and comparative investigation. Diagn Cytopathol 15:33–36
Nagel H, Hemmerlein B, Ruschenburg I, Hüppe K, Droese M (1998) The value of anti-calretinin antibody in the differential diagnosis of normal and reactive mesothelia versus metastatic tumors in effusion cytology. Pathol Res Pract 194:759–764
Shield PW, Callan JJ, Devine PL (1994) Markers for metastatic adenocarcinoma in serous effusion specimens. Diagn Cytopathol 11:237–245
Spehn J, Iwanetz S, Schmitz-Huebner U (1995) Immunozytochemische Untersuchung von Pleuraerguß und Ascites mit Ber-EP4 Antikörpern. DMW 120:1197–1200
Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, Nakanishi Y, Shimoda T (2001) Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer 92:753–760
Morgagni P, Paragoni L, Scarpi E, Zattini PS, Zaccaroni A, Morgagni D, Bazzocchi F (2003) Lymph node micrometastases in early gastric cancer and their impact on prognosis. World J Surg 27:558–561
Greenblatt MS, Bennett WP, Hollstein M, Harris CC (1994) Mutations in the p53 tumour suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 54:4855–4878
Hollsetin M, Sidransky D, Vogelstein B, Harris CC (1991) p53 mutations in human cancer. Science 253:49–53
Kalthoff H, Schmiegel W, Roeder C, Kasche D, Schmidt A, Lauer G, Thiele HG, Honold G, Pantel K, Riethmüller G, Scherer E, Maurer J, Maacke H, Deppert W (1993) p53 and K-ras alterations in pancreatic epithelium cell lesions. Oncogene 8:289–298
Baas IO, Mulder JWR, Offerhaus GJA, Vogelstein B, Hamilton SR (1994) An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol 172:5–12
Werther K, Normark M, Brunner N, Nielsen HJ (2002) Cytokeratin-positive cells in preoperative peripheral blood and bone marrow aspirates of patients with colorectal cancer. Scand J Clin Lab Invest 62:49–57
Broll R, Lembcke K, Stock C, Zingler M, Duchrow M, Schimmelpenning H, Stirk M, Müller G, Kujath P, Bruch HP (1996) Tumorzelldissemination in das Knochenmark und die Peritonealhöhle. Eine immunzytochemische Untersuchung an Patienten mit einem Magen- und kolorektalen Karzinom. Langenbecks Arch Chir 381:51–58
Schlimok G, Funke I, Pantel K, Strobel F, Lindemann F, Witte J, Riethmüller G (1991) Micrometastatic tumour cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer 27:1461–1465
Jauch KW, Heiss MM, Gruetzner U, Funke I, Pantel K, Babic R, Eissner HJ, Riethmüller G, Schildberg FW (1996) Prognostic significance of bone marrow micrometastases in patients with gastric cancer. J Clin Oncol 14:1810–1817
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalogue of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R, Lowe A, Laufer J, Franke WW (1992) Cytokeratin 20 in human carcinomes: a new diagnostic marker by monoclonal antibodies. Am J Pathol 140:427–447
Hermanek P, Hutter P, Sobin L, Wittekind K (1999) Classification of isolated tumor cells and micrometastasis. Cancer 86:2668–26743
Lammers R, Giesert C, Grünebach F, Marxner A, Vogel W, Bühring HJ (2002) Monoclonal antibody 9C4 recognizes epithelial cellular adhesion molecule, a cell surface antigen expressed in early steps of erythropoiesis. Exp Hematol 30:537–545
Knisely AS (1998) Cryptic tumor cells in lymph nodes of patients with esophageal cancer. New Engl J Med 338:550
Bogoevski D, Yekebas EF, Schurr P, Kaifi J, Kutup A, Ebersdobler A, Pantel K, Izbicki J (2004) Mode of spread in the early phase of lymphatic metastasis in pancreatic ductal adenocarcinoma. Ann Surg 240(6):993–1001
Borgen E, Beiske K, Trachsel S, Nesland JM, Kvalhein G, Herstad TK, Schlichting E, Quist H, Naume B (1998) Immunocytochemical detection of isolated epithelial cells in bone marrow: Non-specific staining and contribution by plasma cells directly reactive to alkaline phosphatase. J Pathol 185:427–434
Offner S, Schmaus W, Witter K, Baretton G, Schlimok G, Passlik B, Riethmüller G, Pantel G (1999) p53 gene mutations are not required for early dissemination of cancer cells. Proc Natl Acad Sci USA 96:6942–6946
Dei Tos AP, Doglioni C, Laurino L, Barbareschi M, Fletcher CDM (1993) p53 protein expression in non-neoplastic lesions and benign and malignant neoplasms of soft tissue. Histopathology 22:45–50
Maacke H, Kessler A, Schmiegel W, Roeder C, Vogel I, Deppert W, Kalthoff H (1997) Overexpression of p53 protein during pancreatitis. Br J Cancer 75:1501–1504
Pezzella F, Micklem K, Turley H, Pulford K, Jones M, Kocialkowski S, Delia D, Aiello A, Bicknell R, Smith K, Harris AL, Gatter KC, Mason DY (1994) Antibody for detecting p53 protein by immunohistochemistry in normal tissues. J Clin Pathol 47:592–596
Demeure MJ, Doffek KM, Komorowski RA, Redlich PN, Zhu Y, Erickson BA, Ritch PS, Pitt HA Wilson SD (1998) Molecular metastases in stage I pancreatic cancer: improved survival with adjuvant chemoradiation. Surgery 124:663–669
Stapleton AM, Timme TL, Gousse AE, Li QF, Tobon AA, Kattan MW, Slawin KM, Wheelre TM, Scardino PT, Thompson TC (1997) Primary human prostate cancer cells harboring p53 mutations are clonally expanded in metastases. Clin Cancer Res 3:1389–1397
Acknowledgments
The authors are grateful to T. Supramaniam, K. Wesselhöft, and T. Domeier for tissue preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milsmann, C., Füzesi, L., Heinmöller, E. et al. Morphological and immunohistochemical characterization of isolated tumor cells by p53 status in gastrointestinal tumors. Langenbecks Arch Surg 393, 49–58 (2008). https://doi.org/10.1007/s00423-007-0218-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-007-0218-x