Abstract
Background and aim
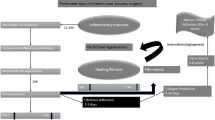
Peritoneal adhesions are caused by intra-abdominal surgery and can lead to relevant complications. Adhesions are supposed to consist of avascular scar tissue. The aim of the present study was to analyze whether mature postsurgical adhesions even after years still reveal a dynamic remodeling process.
Materials and methods
In a prospective analysis, we investigated tissue specimen of peritoneal adhesions in 40 patients after abdominal surgery. Expression of five parameters representing wound healing and remodeling were examined (MMP-2, Ki-67, apoptosis, collagen/protein ratio, and collagen type I/III ratio).
Results
Gender, age, and the number of previous operations had no impact on the parameters measured. Adhesion specimens were cell rich, containing mononuclear round cells, fibroblasts, adipose cells, and vascular endothelial cells. There was a positive expression of MMP-2 and apoptosis, whereas Ki-67 was marginal irrespective of adhesion maturity or quality. Adhesions classified as dense showed a significant increase in total collagen (118.2 ± 4.9 μg/mg) and collagen type I/III ratios (3.9 ± 0.2), whereas there were no significant differences regarding the adhesion maturity.
Conclusion
The distinct composition of cellular components as well as of extracellular matrix proteins may reflect an interactive cross-talk between adhesion- and stroma-derived cells even in mature adhesions. Our findings support the hypothesis that the disabilities of appropriate repair of the peritoneal surface leading to persistent adhesions are a consequence of a permanent process of disturbed remodeling.



Similar content being viewed by others
References
Operative Laparoscopy Study Group (1991) Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Operative Laparoscopy Study Group. Fertil Steril 55:700–704
Ballas CB, Davidson JM (2001) Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair Regen 9:223–237
Conze J, Rosch R, Klinge U, Weiss C, Anurov M, Titkowa S, Oettinger A, Schumpelick V (2004) Polypropylene in the intra-abdominal position: influence of pore size and surface area. Hernia 8:365–372
DeCherney AH, diZerega GS (1997) Clinical problem of intraperitoneal postsurgical adhesion formation following general surgery and the use of adhesion prevention barriers. Surg Clin North Am 77:671–688
diZerega GS (1994) Contemporary adhesion prevention. Fertil Steril 61:219–235
Ellis H (1962) Post-operative intra-abdominal adhesions. Nature 194:580–581
Ellis H (1962) The aetiology of post-operative abdominal adhesions. An experimental study. Br J Surg 50:10–16
Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O’Brien F, Buchan S, Crowe AM (1999) Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet 353:1476–1480
Epstein JC, Wilson MS, Wilkosz S, Ireland G, O’Dwyer ST, Herrick SE (2006) Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis Colon Rectum 49:1885–1892
Helary C, Ovtracht L, Coulomb B, Godeau G, Giraud-Guille MM (2006) Dense fibrillar collagen matrices: a model to study myofibroblast behaviour during wound healing. Biomaterials 27:4443–4452
Herrick SE, Mutsaers SE, Ozua P, Sulaiman H, Omer A, Boulos P, Foster ML, Laurent GJ (2000) Human peritoneal adhesions are highly cellular, innervated, and vascularized. J Pathol 192:67–72
Inkinen K, Turakainen H, Wolff H, Ravanti L, Kahari VM, Ahonen J (2000) Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. Acta Pathol Microbiol Immunol Scand 108:318–328
Jenkins SD, Klamer TW, Parteka JJ, Condon RE (1983) A comparison of prosthetic materials used to repair abdominal wall defects. Surgery 94:392–398
Jirasek JE, Henzl MR, Uher J (1998) Periovarian peritoneal adhesions in women with endometriosis. Structural patterns. J Reprod Med 43:276–280
Junge K, Klinge U, Rosch R, Mertens PR, Kirch J, Klosterhalfen B, Lynen P, Schumpelick V (2004) Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbeck’s Arch Surg 389:17–22
Junqueira LC, Cossermelli W, Brentani R (1978) Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41:267–274
Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL (2001) Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg 18:260–273
Lopez-De Leon A, Rojkind M (1985) A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem 33:737–743
Menzies D (1992) Peritoneal adhesions. Incidence, cause, and prevention. Surg Annu 24(Pt 1):27–45
Menzies D, Ellis H (1990) Intestinal obstruction from adhesions-how big is the problem? Ann R Coll Surg Engl 72:60–63
Monk BJ, Berman ML, Montz FJ (1994) Adhesions after extensive gynecologic surgery: clinical significance, etiology, and prevention. Am J Obstet Gynecol 170:1396–1403
Muller SA, Treutner KH, Tietze L, Anurov M, Titkova S, Polivoda M, Oettinger AP, Schumpelick V (2001) Influence of intraperitoneal phospholipid dosage on adhesion formation and wound healing at different intervals after surgery. Langenbecks’ Arch Surg 386:278–284
Ravanti L, Kahari VM (2000) Matrix metalloproteinases in wound repair (review). Int J Mol Med 6:391–407
Ray NF, Denton WG, Thamer M, Henderson SC, Perry S (1998) Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg 186:1–9
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8:138–140
Rout UK, Saed GM, Diamond MP (2005) Expression pattern and regulation of genes differ between fibroblasts of adhesion and normal human peritoneum. Reprod Biol Endocrinol 3:1
Roy S, Clark CJ, Mohebali K, Bhatt U, Wallace WA, Nahman NS, Ellison EC, Melvin WS, Sen CK (2004) Reactive oxygen species and EGR-1 gene expression in surgical postoperative peritoneal adhesions. World J Surg 28:316–320
Saed GM, Munkarah AR, bu-Soud HM, Diamond MP (2005) Hypoxia upregulates cyclooxygenase-2 and prostaglandin E(2) levels in human peritoneal fibroblasts. Fertil Steril 83(Suppl 1):1216–1219
Thompson J (1998) Pathogenesis and prevention of adhesion formation. Dig Surg 15:153–157
Treutner KH, Bertram P, Loser S, Winkeltau G, Schumpelick V (1995) Prevention and therapy of intra-abdominal adhesions. A survey of 1,200 clinics in Germany. Chirurg 66:398–403
Treutner KH, Schumpelick V (2000) Prevention of adhesions. Wish and reality. Chirurg 71:510–517
Van Der Krabben AA, Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, Schaapveld M, Van GH (2000) Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg 87:467–471
Wilson MS, Ellis H, Menzies D, Moran BJ, Parker MC, Thompson JN (1999) A review of the management of small bowel obstruction. Members of the Surgical and Clinical Adhesions Research Study (SCAR). Ann R Coll Surg Engl 81:320–328
Acknowledgments
We are grateful to Mrs. Ellen Krott for most excellent and careful assistance during this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Binnebösel, M., Klinge, U., Rosch, R. et al. Morphology, quality, and composition in mature human peritoneal adhesions. Langenbecks Arch Surg 393, 59–66 (2008). https://doi.org/10.1007/s00423-007-0198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-007-0198-x