Abstract
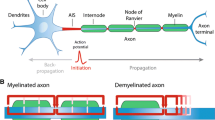
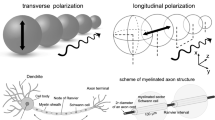
Ectopic activity in multiple sclerosis (MS) patients has been traditionally attributed to hyperexcitability of the demyelinated axon segments. Here, we propose that the same outcome may be the result of persistent reflection—the continuous reactivation of the axonal nodes that limit a demyelinated internodal segment. Using computer simulations, we studied the patterns of impulse propagation for a wide range of electrophysiological conditions. In uniformly myelinated fibers, increasing the temperature enabled successful propagation with no blocks in more severe conditions of demyelination. Secondary activations that were originated at the paranodes were formed for temperatures lower than T = 305 K, and at the condition of high sodium channel excitability. Non-sustained and persistent reflections appeared in the case of focally demyelinated fibers, and only within a narrow range of parameters of high temperature and membrane excitability. Persistent reflection reached steady state in ionic currents within 4 ms, and was characterized with a very high activation frequency of 1.504 ± 0.039 kHz. We conclude that persistent reflection is a possible mechanism for ectopic activity in MS patients, being more prominent in higher temperatures and severe axonal demyelination. Eliminating these symptoms may be addressed by cooling the body or by applying pharmacological agents to alter excitability properties.
Similar content being viewed by others
References
Amir R, Devor M (2003) Extra spike formation in sensory neurons and the disruption of afferent spike patterning. Biophys J 84: 2700–2708
Amir R, Liu CN, Kocsis JD, Devor M (2002a) Oscillatory mechanism in primary sensory neurons. Brain 125: 421–435
Amir R, Michaelis M, Devor M (2002b) Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by developing afterpotentials. J Neurosci 22: 1187–1198
Baccus SA, Burrell BD, Sahley CL, Muller KJ (2000) Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J Neurophysiol 83: 1693–1700
Baker M, Bostock H (1992) Ectopic activity in demyelinated spinal root axons of the rat. J Physiol 451: 539–552
Bergmans JA (1983) Neuorophysiological features of experimental and human neuropathies. In: Battistin L, Hashim GA, Lajtha A (eds) Clinical and biological aspects of peripheral nerve diseases. Liss, New York, pp 73–100
Cabo C, Barr RC (1992) Reflection after delayed excitation in a computer model of a single fiber. Circ Res 71: 260–270
Compston A, Coles A (2002) Multiple sclerosis. Lancet 359: 1221–1231
Goldman L, Albus JS (1968) Computation of impulse conduction in myelinated fibers; theoretical basis of the velocity–diameter relation. Biophys J 8: 596–607
Goldstein SS, Rall W (1974) Changes of action potential shape and velocity for changing core conductor geometry. Biophys J 14: 731–757
Halter JA, Clark JW Jr (1991) A distributed-parameter model of the myelinated nerve fiber. J Theor Biol 148: 345–382
Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R (2007) How common are the “common” neurologic disorders. Neurology 68: 326–337
Howe JF, Calvin WH, Loeser JD (1976) Impulses reflected from dorsal root ganglia and from focal nerve injuries. Brain Res 116: 139–144
Jacobsen EW, Cedersund G (2008) Structural robustness of biochemical network models—with application to the oscillatory metabolism of activated neutrophils. IET Syst Biol 2: 39–47
Jalife J, Moe GK (1981) Excitation, conduction, and reflection of impulses in isolated bovine and serum cardiac purkinje fibers. Circ Res 49: 233–247
Kapoor R, Smith KJ, Felts PA, Davies M (1993) Internodal potassium currents can generate ectopic impulses in mammalian myelinated axons. Brain Res 611: 165–169
Kapoor R, Li YG, Smith KJ (1997) Slow sodium-dependent potential oscillations contribute to ectopic firing in mammalian demyelinated axons. Brain 120: 647–652
Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E (1999) TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem 274: 1381–1387
Matthews WB (1975) Paroxysmal symptoms in multiple sclerosis. J Neurol Neurosurg Psychiatry 38: 617–623
McDonald WI, Ron MA (1999) Multiple sclerosis: the disease and its manifestations. Philos Trans R Soc Lond B 354: 1615–1622
Nordin M, Nystrom B, Wallin U, Hagbarth KE (1984) Ectopic sensory discharges and paresthesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain 20: 231–245
Nygren A, Halter JA (1999) A general approach to modeling conduction and concentration dynamics in excitable cells of concentric cylindrical geometry. J Theor Biol 199: 329–358
Reutskiy S, Rossoni E, Tirozzi B (2003) Conduction in bundles of demyelinated nerve fibers: computer simulation. Biol Cybern 89: 439–448
Rudy Y (2005) Lessons learned about slow discontinuous conduction from models of impulse propagation. J Electrocardiol 38: 52–54
Schwarz JR, Eikhof G (1987) Na currents and action potentials in rat myelinated nerve fibres at 20 and 37 degrees C. Pflugers Arch 409: 569–577
Schwieler JH, Zlochiver S, Pandit SV, Berenfeld O, Jalife J, Bergfeldt L (2008) Reentry in an accessory atrioventricular pathway as a trigger for atrial fibrillation initiation in manifest Wolff-Parkinson-White syndrome: a matter of reflection? Heart Rhythm 5: 1238–1247
Smith KJ, McDonald WI (1999) The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B 354: 1649–1673
Stephanova DI, Bostock H (1995) A distributed-parameter model of the myelinated human motor nerve fibre: temporal and spatial distributions of action potentials and ionic currents. Biol Cybern 73: 275–280
Utzschneider D, Kocsis J, Devor M (1992) Mutual excitation among dorsal root ganglion neurons in the rat. Neurosci Lett 146: 53–56
Waxman SG, Ritchie JM (1993) Molecular dissection of the myelinated axon. Ann Neurol 33: 121–136
Wu N, Hsiao CF, Chandler SH (2001) Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation. J Neurosci 21: 3729–3739
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zlochiver, S. Persistent reflection underlies ectopic activity in multiple sclerosis: a numerical study. Biol Cybern 102, 181–196 (2010). https://doi.org/10.1007/s00422-009-0361-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-009-0361-2