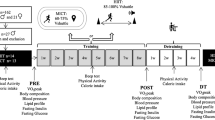
Abstract
Purpose
This study compared the effects of polarized running training adapted to the menstrual cycle (MC) phases versus polarized training adapted contrary to the MC on endurance performance and cardiovascular parameters.
Methods
Thirty-three naturally menstruating, moderately trained females (age: 26 ± 4 years; BMI: 22.3 ± 3.2 kg/m2; \(\dot{V}\)O2max/rel: 40.35 ± 4.61 ml/min/kg) were randomly assigned to a control (CON) and intervention (INT) group. Both groups participated in a load-matched eight-week running training intervention. In the INT, high-intensity sessions were aligned with the mid and late follicular phase, low-intensity sessions with the early and mid-luteal phase, and recovery with the late luteal and early follicular phase. In the CON, high-intensity sessions were matched to the late luteal and early follicular phase, and recovery to the mid and late follicular phase. Endurance performance and cardiovascular parameters were assessed at baseline and after the intervention.
Results
Twenty-six females completed the intervention. A repeated measures ANOVA determined no time × group interaction effect for any parameter. A significant time effect was found for maximal oxygen uptake (F(1,12) = 18.753, p = 0.005, ηp2 = 0.630), the velocity at the ventilatory threshold one (F(1,12) = 10.704, p = 0.007, ηp2 = 0.493) and two (F(1,12) = 7.746, p = .018, ηp2 = .413).
Conclusion
The training intervention improved endurance performance in both groups, with no further benefit observed from the MC-adapted polarized training in a group-based analysis. Replications with an extended intervention period, a larger sample size, and a more reliable MC determination are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout a female's life, from menarche to the onset of menopause, a regular biological rhythm known as the menstrual cycle (MC) governs the ebb and flow of endogenous sex hormones, particularly estrogen and progesterone (de Jonge et al. 2019). These hormonal fluctuations orchestrate the reproductive system's function while exerting influence over other physiological systems, including the cardiovascular, respiratory, and nervous systems (Bernstein and Behringer 2023).
Variations in the concentrations of these hormones, specifically estrogens and progesterone, may have further implications for athletic performance (McNulty et al. 2020). Even though isolating single actions of hormones due to interdependency is challenging, estrogens and progesterone exhibit distinct influences on the energy metabolism in naturally menstruating females. Estrogens appear to impact the oxidation of energy substrates, leading to an increased rate of carbohydrate oxidation (Zderic et al. 2001) and a higher utilization rate of glycogen oxidation (Devries 2016), ultimately favoring increased endurance performance (Bernstein and Behringer 2023). On the other hand, progesterone, acting as an estrogen antagonist, inhibits carbohydrate oxidation, resulting in increased protein catabolism and higher rates of amino acid oxidation (Boisseau and Isacco 2021). Furthermore, during the luteal phase, which is characterized by elevated progesterone concentrations, an increase in muscle glycogen-sparing effects (Devries 2016; Oosthuyse and Bosch 2010), and an enhanced reliance on lipid metabolism can be observed (Oosthuyse and Bosch 2010; Willett et al. 2021). These metabolic variations may affect individual training readiness and overall training responses throughout the MC.
Even though general guidelines on modulating exercise training according to the MC do not exist, adapting the training to the MC may alter long-term performance development (Recacha-Ponce et al. 2023). While previous research reports a positive impact of adjusting resistance training variables according to the MC on different performance outcomes (Kissow et al. 2022), there remains a notable gap in knowledge regarding endurance training. To our knowledge, only one previous publication from our research group explored this topic (Kubica et al. 2023). In this study, we compared the effects of a traditional eight-week block periodized running training with a polarized running training adapted to the MC phases on a range of variables, including endurance performance, cardiovascular parameters, recovery, and MC-related symptoms. A major limitation of this study was that, although randomly allocated to intervention and control groups, a significant portion of the control group's training coincidentally aligned with the MC phases, leading to only minor differences in the training protocols between the groups. This made it challenging to identify any potential influences of the MC.
Considering the limitations of the previous study and lack of research in the field, the primary study adopts an exploratory approach to compare the effects of polarized training adapted to the MC phases with polarized training explicitly adapted in contrast to the MC phases on endurance performance and cardiovascular parameters in naturally menstruating females. By devising distinct training interventions, we aim to gain a clearer understanding of the impact of MC-adapted running training on endurance performance and cardiovascular outcomes.
Methods
Study design and participants
A parallel arm randomized controlled design involving forty moderately active females was implemented. Participants were eligible to take part in the study if they met the following criteria: (1) had a regular menstrual cycle length (between 21 and 35 days with no more than ± 3 days of variation within the last 6 months), (2) were not pregnant (last pregnancy > 1 year), (3) were not using hormonal contraceptives (not within the last 6 months), (4) had no underlying health conditions or orthopedic injuries, (5) exhibited ovulation, and (6) met the minimum physical activity requirements (> 150 min of moderate/vigorous physical activity per week with at least 15 min of vigorous physical activity per week).
Participants were recruited via announcements at the University of Bern, personal contact, and social media posts. The recruitment and data collection period spanned from December 2022 to April 2023.
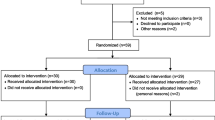
Seven participants did not meet the inclusion criteria and were subsequently excluded from the study (Fig. 1 Flow Chart). A total of 33 participants (age: 26 ± 4 years; body mass index (BMI): 22.3 ± 3.2 kg/m2) were randomly assigned either to a control group (CON) or an intervention group (INT) (Fig. 2). The principal investigator conducted the randomization using a computer-generated random number table.
Each participant provided written, informed consent after being presented with an explanation of the study's objectives and the experimental procedures. This study received approval from the Ethical Commission of the Faculty of Human Sciences at the University of Bern (Nr. 2022-01-00006).
Measurements
Participants underwent a baseline assessment, an 8-week intervention, and a post assessment. Both testing sessions occurred at the Institute of Sport Science of the University of Bern on the same time of day at the same day of the week to minimize circadian fluctuations. For each testing session, participants were asked to be at least 2 h postprandial, refrain from consuming caffeine and alcohol for a minimum of 4 h, and avoid exercising for at least 48 h. All measurements were conducted by well-trained research staff using standardized equipment and procedures in controlled conditions. The measurements during the baseline and post-assessments were conducted in the following order: demographics and anthropometrics, cardiovascular parameters, and endurance performance.
Demographics and anthropometrics
Demographic data, medical history, and premenstrual symptoms were obtained through questionnaires. To assess premenstrual symptoms, the German version of the screening instrument for premenstrual symptoms (SIPS) (Bentz et al. 2012) was administered once during the baseline assessment.
Standing height and body mass were determined using a stadiometer and a body composition scale (RD 545HR, Tanita Europe BV, Amsterdam, Netherlands). Waist circumference was measured with non-elastic tape at the midpoint between the costal arch and the upper edge of the iliac crest. Body Mass Index (BMI) was then computed based on weight in kilograms and height in meters (kg/m2). The Waist-to-Height Ratio (WHtR) was determined by dividing waist circumference by height (waist circumference/height).
Endurance performance
A graded exercise test on a treadmill ergometer (h/p/cosmos pulsar 4.0; h/p/cosmos sports and medical GmbH, Nussdorf-Traunstein, Germany) was conducted to assess maximal oxygen consumption (\(\dot{V}\)O2max). The test started with an initial speed of 6.6 km h−1, and increased stepwise by 1.2 km h−1 every 3 min, with 30 s of passive rest after each step until volitional exhaustion was achieved.
Oxygen consumption was continuously monitored using a breath-by-breath gas analyser (Metalyzer 3B, Cortex, Leipzig, Germany). \(\dot{V}\)O2max was computed as the highest recorded value, using a rolling average of 15-s intervals. To ensure the accuracy of the measurements, a two-point calibration procedure was performed prior to each test day. This procedure involved calibrating the oxygen and carbon dioxide sensors with gases of known concentrations and calibrating the flow rate using a 3-L syringe. Additionally, ambient air calibration was conducted before each test.
To confirm the attainment of \(\dot{V}\)O2max, at least three of the following criteria had to be met: (1) a final rating of perceived exertion score of ≥ 17 on the Borg scale (scale 6–20), (2) a respiratory exchange ratio > 1.1, (3) no further change in heart rate (HR) with an increase in workload, (4) a "plateau" (an increase of ≤ 150 ml) in oxygen uptake with a simultaneous increase in workload, (5) volitional fatigue.
Two investigators independently identified ventilatory thresholds (VT1 and VT2). If there was a lack of agreement, the opinion of a third observer was sought. VT1 was defined as the workload at which increases were observed in the ventilatory equivalent for oxygen and the end-tidal pressure of oxygen without a simultaneous rise in the ventilatory equivalent for carbon dioxide. Similarly, VT2 was determined when increases were evident in the ventilatory equivalent for oxygen and the ventilatory equivalent for carbon dioxide, accompanied by a reduction in the end-tidal pressure of carbon dioxide (Amann et al. 2004; Gaskill et al. 2001).
HR was continuously recorded beat-to-beat throughout the graded exercise test using a Polar HR sensor (H10, Polar Electro Oy, Kempele, Finland).
Cardiovascular parameters
Hemodynamics
Peripheral systolic blood pressure (BP), peripheral diastolic BP, and pulse wave velocity (PWV) were assessed utilizing the Mobil-O-Graph® (PWA-Monitor, IEM, Stollberg, Germany), a clinically validated device (Franssen and Imholz 2010). Two measurements were taken on the upper right arm using customized arm cuffs following 10 min of supine rest. The mean of the two measurements was used for analysis.
Heart rate variability (HRV)
Heart rate variability (HRV) was assessed using a HR sensor and a chest strap (Polar RS800 CX®, Polar Electro OY, Kempele, Finland). Following 5 min of rest in a supine position, a 5-min measurement was conducted. Throughout the measurement, patients were instructed to maintain a normal and comfortable breathing pattern.
The raw data underwent processing using the app Elite HRV (Elite HRV Inc, 2022), which has demonstrated validity and reliability (Moya-Ramon et al. 2022). The analysis included the assessment of the root mean square of successive RR interval differences (RMSSD), the standard deviation of NN intervals (SDNN), and the resting HR (restHR).
Determination of the MC and cycle phasing
A month before the intervention, a three-step verification process was employed to confirm that participants met the inclusion and exclusion criteria and to ascertain their MC phases during the intervention. Furthermore, it was examined whether the MC characteristics of the participants aligned with the definition of naturally menstruating females (Elliott-Sale et al. 2021). This three-step verification encompassed the following:
-
1.
Calendar-based counting: Individuals tracked and recorded the onset and duration of their menstrual periods on a calendar, using historical data to calculate the approximate dates for future MC.
-
2.
Measurement of basal body temperature: Each morning, upon waking, participants measured their basal body temperature using the Breuer FT 09 thermometer (Breuer GmbH, Ulm, Germany).
-
3.
Ovulation: Participants were provided with ovulation tests (Pinkline Ovulation Test 25 mlU/mL, Pinkline By Burggraf, Taverne, Switzerland) with the recommended threshold of 25 mlU/mL (Leiva et al. 2017). These kits involved colorimetric enzyme immunoassays of urinary luteinizing hormone. Participants were instructed to conduct the ovulation tests according to the manufacturer's directions. Further, ovulation tests were performed from the seventh day of the MC onwards. Ovulation was assumed to have occurred one day after a positive ovulation test. If no positive ovulation test was recorded during the MC, testing was postponed for one more cycle until a positive ovulation test was observed. Participants were excluded from the study if they experienced two consecutive cycles without a positive ovulation test (s. Fig. 1).
MC phases were calculated according to the recommendation from Schmalenberger et al. (2021) as follows:
-
Mid to late follicular phase including periovulation: + 4 days after menstrual onset until periovulation + 1 day following a positive ovulation test and nadir.
-
Early luteal–mid-luteal phase: + 2 days following a positive ovulation test and nadir until + 10 days following a positive ovulation test and nadir.
-
Luteal phase and menstruation/early follicular phase: − 3 days before onset of bleeding until + 4 days after menstrual onset.
Training protocol
The training protocol consisted of an 8-week running training intervention, including three weekly training sessions. Both groups followed a general polarized running training program, which is recognized an effective training approach for recreationally active runners (Muñoz et al. 2014). The program was designed to attain a total percentage distribution in training zones 1, 2, and 3 of 75%/5%/20% based on HR distribution and running velocity at the VT1 and VT2. In the INT, the single running training sessions were adapted according to current MC training recommendations (Elliott-Sale and Pitchers 2019). Running training sessions including Zone 3 training, were mainly performed during the mid and late follicular phase, matched with the parallel increase in oestrogen concentration, which presumably improves the oxidation of carbohydrates and the uptake of glycogen into type I muscle fibres (Hackney 2021). Running training sessions, including Zone 1 and 2, were mainly performed during the early and mid-luteal phases, characterized by high levels of progesterone, which is suggested to increase the reliance on fat metabolism (Hackney 2021). During the premenstrual and menstrual phases, only running training sessions, including Zone 1 with a reduced volume, were performed (Elliott-Sale and Pitchers 2019; Recacha-Ponce et al. 2023). The CON followed the same running training program. However, the single running training sessions were contrary adapted to their MC. Running training sessions, including Zone 3 training, were mainly performed during the premenstrual and menstrual phases. Zone 1 running training with a reduced volume was performed during the mid and late follicular phase. Running training during the early and mid-luteal phase was comparable to the INT, with training sessions in Zone 1 and 2. We chose to maintain a consistent training frequency throughout the intervention duration for both the INT and CON groups, while varying the intensity of the training intervention. This decision was made to mitigate the potential risk of injuries associated with fluctuating training frequencies, as suggested by Ferreira et al. (2012).
Both interventions were designed to have equivalent workloads and were HR controlled (H10, Polar Electro OY, Kempele, Finland). Participants were instructed to record their training frequency, volume, and average training HR in a digital diary (m-path application, KU Leuven R&D, Leuven, Belgium). Participants who missed more than three of the 24 training sessions were excluded from the analysis.
Statistics
We analyzed data using IBM SPSS Statistics for Windows, Version 27.0 (IBM Corp. Released 2020, Armonk, NY, USA). The results are reported as means ± standard deviation. To assess differences in subject characteristics between the groups at baseline, we employed independent samples t tests. Repeated measures analysis of variance (ANOVA) was utilized to investigate the interactions between time × group regarding the outcomes. Post-hoc analyses with Bonferroni's correction were conducted if appropriate. ANOVA effect sizes (partial eta squared (ηp2)) are defined as small, medium, and large: 0.01 to ≤ 0.06, 0.06 to < 0.14, and ≥ 0.14, respectively (Richardson 2011). The effect size for the t tests was measured by Cohen's d (d). Small, medium, and large effect sizes were designated as ¦d¦ = 0.2, ¦d¦ = 0.5, and ¦d¦ = 0.8, respectively (Cohen 2013). Statistical significance was set a priori at p < 0.05.
To determine the reliability of the variables, intraclass correlation coefficients (ICC) and their 95% confidence intervals were calculated based on a mean rating, consistent, 2-way mixed-effects model (Koo and Li 2016; Weir 2005). ICC values less than 0.5 indicate poor reliability, values between 0.5 and 0.75 reveal moderate reliability, values between 0.75 and 0.9 reveal good reliability, and values greater than 0.90 reveal excellent reliability (Portney and Watkins 2009). Furthermore, standard error of measurement (SEM) and minimum difference to be considered real (MD) were calculated as follows (Weir 2005):
Results
Participants' characteristics
No adverse events were recorded during the assessments for any of the patients. Seven participants (INT = 3, CON = 4) were lost before post-assessments due to various reasons, as indicated in Fig. 1. Consequently, fourteen females from the INT (age: 26 ± 4; BMI: 21.7 ± 2.8 kg/m2; \(\dot{V}\)O2max/M: 39.9 ± 4.6 mL min−1 kg−1) and twelve females from the CON (age: 26 ± 3; BMI: 22 ± 2.2 kg m−2; \(\dot{V}\)O2max/M 40.8 ± 4.8 mL min−1 kg−1) were incorporated in the final analysis (Table 1). No significant group differences (p < 0.05) were identified in the baseline assessment for all parameters.
Based on BMI, two participants from the INT and one from the CON were classified as underweight (Weisell 2002). According to the WHtR cut-off point of 0.5, all participants were within the healthy range and not characterized as central obese (Yoo 2016). Based on peripheral BP results, two participants in the CON and one in the INT were classified as hypertensive (Flack and Adekola 2020). Regarding \(\dot{V}\)O2max/M, participants in the CON were categorized as follows: three were untrained, and nine were active. In the INT, six participants were considered untrained, and eight as active (Decroix et al. 2016). According to the SIPS and following the criteria established by Bentz et al. (2012), six participants in the CON and seven in the INT had premenstrual symptoms
INT intervention group, CON control group, MC length average menstrual cycle length in the previous three MCs, BMI body mass index, WHtR Waist-to-Height-Ratio
Endurance performance
We found no statistically significant difference in endurance parameters according to the groups over time (vVT1 (F(1,12) = 0.153, p = 0.703, ηp2 = 0.014), hrVT1 (F(1,12) = 3.039, p = 0.109, ηp2 = 0.216), vVT2 (F(1,12) = 0.409, p = 0.535, ηp2 = 0.036), hrVT2 (F(1,12) = 2.521, p = 0.141, ηp2 = 0.186); and \(\dot{V}\)O2max/M (F(1,12) = 1.017, p = 0.335, ηp2 = 0.850) (s. Table 2).
However, a significant time effect was found for \(\dot{V}\)O2max/M (F(1,12) = 18.753, p = 0.005, ηp2 = 0.630), vVT1 (F(1,12) = 10.704, p = 0.007, ηp2 = 0.493) and vVT2 (F(1,12) = 7.746, p = 0.018, ηp2 = 0.413), but not for the other endurance parameters. No group effects were found for any of the endurance parameters
Cardiovascular parameters
No significant time × group interactions effects were found for the cardiovascular parameters (systolic BP (F(1,12) = 2.092, p = 0.176, ηp2 = 0.160), diastolic BP (F(1,12) = 0.144, p = 0.711, ηp2 = 0.013), restHR (F(1,12) = 0.001, p = 0.979, ηp2 = 0.000), RMSSD (F(1,12) = 2.617, p = 0.134, ηp2 = 0.192) and SDNN (F(1,12) = 1.122, p = 0.312, ηp2 = 0.093)) (Table 3). Additionally, no significant time effects were found for any of the cardiovascular parameters.
ICC, SEM and MD
The ICC, SEM, MD, and the proportion of participants with changes exceeding the MD are reported in Table 4. The outcomes showed poor reliability for vVT1, systolic BP, diastolic BP, and PWV, and moderate reliability for hrVT1, vVT2, hrVT2, \(\dot{V}\)O2max/M, restHR, RMSSD and SDNN. Based on the ICC and SEM, the following MD resulted for the performance parameters: vVT1: 4.2 km h−1, hrVT1: 21.5 b min−1, vVT2: 1.7 km h−1, hrVT2: 14.2 m min−1, \(\dot{V}\)O2max/M: 7.0 mL min−1 kg−1; and for the cardiovascular parameters: systolic BP: 21.5 mmHg, diastolic BP: 12.2 mmHg, PWV: 0.7 m s−1, restHR: 16.5 b min−1, RMSSD: 7.0 ms, and SDNN: 230 ms.
Individual patterns of training responses for endurance and cardiovascular parameters following 8 weeks of running training are displayed in Fig. 2.
Individual patterns of training responses based on MD. Individual patterns of response following eight weeks of training. Positive responses with an individual change from baseline to post-assessment larger than the MD (black boxes), minor responses smaller than the MD (negative minor change-scattered boxes and positive minor change—white boxes), and adverse responses exceeding the MD (grey boxes) are shown for all participants across all variables. MD minimal difference to be considered real, MD% percentage of participants with a difference ≥ MD, INT intervention group, CON control group, vVT1 velocity at ventilatory threshold 1, hrVT1 heart rate at ventilatory threshold 1, vVT2 velocity at ventilatory threshold 2, hrVT2 heart rate at ventilatory threshold 2, \(\dot{V}\)O2max/M maximal oxygen consumption normalized per body mass, BP blood pressure, PWV pulse wave velocity, restHR resting heart rate, RMSSD root mean square of successive RR interval differences, SDNN Standard Deviation of NN Interval, PMS premenstrual Syndrome
Discussion
In this study, our primary objective was to assess the effects of two distinct training approaches—polarized training adapted to the MC and anti-MC-periodized training—on the endurance performance and cardiovascular parameters of moderately active females.
Endurance performance
Both training programs significantly improved aerobic capacity and running velocity at the ventilatory thresholds. However, our findings revealed no significant differences between the two training approaches.
This indicates that, for moderately active females, adapting running training to the MC does not confer a substantial performance advantage. These results align with earlier findings from our research group (Kubica et al. 2023). A major limitation of the previous study was that a significant portion of the control group's training coincidentally aligned with the MC phases. In the current study, we therefore implemented a polarized training adapted contrary to the MC in the CON to increase the differences between the training protocols. Despite these adjustments in the study design, no differences were observed between the groups.
Unfortunately, limited research on the effect of MC-adapted endurance training makes it difficult to classify the current results further. However, previous research on resistance training indicates a positive effect of adapting the training to the MC (Kissow et al. 2022; Thompson et al. 2020). It has been suggested that metabolic shifts during the MC may alter training readiness and response (Devries 2016; Hackney 2021; Hackney et al. 2021; Isacco and Boisseau 2017; Vigh-Larsen et al. 2021). However, the current body of literature remains inconsistent (Hulton et al. 2021). The inconsistent results may be attributed to factors such as training intensity (Oosthuyse and Bosch 2010) and nutritional status (Hulton et al. 2021; McLay et al. 2007), which are often not adequately controlled in research studies. Those factors could potentially play a more influential role in metabolism and thus have a more substantial impact on training readiness and response than the MC. In the present study, we controlled the training intensity using HR. Unfortunately, the diet was not fully controlled. Participants were only encouraged to maintain adequate carbohydrate intake before each training session. Thus, it cannot be ruled out that differences in nutritional status before the exercises could have mitigated potential metabolic shifts associated with the MC (Hulton et al. 2021).
Apart from the energy metabolism itself, various other factors, including individual athlete perceptions of the MC's impact on training and performance, as well as the lived experiences and stigmas related to the MC, can also influence training readiness and responses (Carmichael et al. 2021; Kolić et al. 2021). Additionally, depending on the exercise intensity, psychological responses to training seem to be altered during the MC (Prado et al. 2021). Those alterations in motivation and affective response might impact training adherence and, therefore, long-term development (Prado et al. 2021). The complexity of overall performance dynamics (Coffey and Hawley 2007), the influence of psychological responses (Prado et al. 2021), compounded by individual variability in how MC phases may affect performance (Julian et al. 2017), makes it crucial to consider individual changes.
Therefore, we examined individual responses using the MD. Notably, in the INT, 31% of females exhibited changes in \(\dot{V}\)O2max/M that exceeded the MD, compared to 8% in the CON. This indicates that the training intervention adapted to the MC resulted in a higher responder rate. However, it is noteworthy that three-quarters of the responders from the INT group and half from the CON group were initially categorized as "unfit," with an initial \(\dot{V}\)O2max/M < 37 ml/min/kg (Decroix et al. 2016). This finding implies a potential influence of the participants' initial fitness levels on the training response, which should be addressed in future studies with larger sample sizes. For all other endurance parameters, changes exceeding the MD were less common in both groups.
According to the literature, the presence of PMS in active females might also impact individual training responses and lead to MC-based performance changes (Carmichael et al. 2021). Therefore, a subgroup analysis of females with and without PMS is recommended to explore the potential effects of PMS and to identify responders and non-responders (Carmichael et al. 2021). However, a statistical sub-group analysis was not feasible due to the small sample size and lack of power. Still, we were not able to identify a pattern when looking at the MD for each outcome and participant and the influence of PMS (s. Fig. 2). Therefore, we assume that PMS had no impact on our results.
In summary, both training approaches led to significant improvements in aerobic capacity and running velocity at the ventilatory thresholds; however, the MC-adapted and non-adapted training did not yield discernible differences in specific performance parameters. Also, the individual responses underscore the complexity and individuality of performance responses to MC-adapted endurance training. In light of our non-significant findings and considering prior research on MC-adapted endurance training (Kubica et al. 2023), it seems plausible to suggest that MC-adapted training may not confer discernible performance benefits in healthy, moderately active, naturally menstruating, young adult females. Nevertheless, further investigation is needed to validate and refine this preliminary conclusion.
Cardiovascular parameters
Besides endurance performance, our study investigated the impact of running training, adapted to and contrary to the MC, on various cardiovascular parameters. The results showed no significant interaction effects or time-related changes in all cardiovascular parameters.
To the best of our knowledge, limited research has explored the impact of MC-adapted endurance training on cardiovascular parameters. The current findings align with a previous investigation from our research group, which demonstrated that MC-adapted endurance training did not yield substantial benefits regarding cardiovascular parameters (Kubica et al. 2023).
When comparing the current results with research on the general influence of endurance training on cardiovascular parameters, the absence of time-related changes in our study contradicts with previous research (Cornelissen and Smart 2013; Reimers et al. 2018). Current meta-analyses indicate a positive effect of endurance training on cardiovascular parameters, specifically by a reduction in BP and restHR (Cornelissen and Smart 2013; Reimers et al. 2018). The recent meta-analyses point out that, on average, females experience a HR reduction of approximately 3.8 bpm following endurance exercise interventions (Reimers et al. 2018). Our results demonstrated comparable but non-significant changes in restHR, with an average decrease of 3.8 bpm in the INT and 3.7 bpm in the CON. However, it is essential to recognize that the intervention duration in our study falls on the lower end of the spectrum compared to the wide range of intervention durations in the meta-analysis (6–104 weeks) (Reimers et al. 2018). This may explain the lack of a significant effect on restHR in our study.
Also, no significant time-related effects were found for systolic BP and diastolic BP in our study. Previous research on the effect of MC-adapted endurance training on BP is limited. To our knowledge, only one previous study from our research group (Kubica et al. 2023) investigated the effects, with no effects of the MC-adapted endurance training as well as block-periodized endurance training led on BP. However, a meta-analysis by Cornelissen et al. (2013) reported that endurance training reduces systolic BP but not diastolic BP. Notably, the effects on systolic BP are more pronounced in individuals with prehypertension or hypertension. Given that our participant group was predominantly healthy, the non-significant changes in systolic BP may be attributed to their baseline health status (Cornelissen et al. 2013).
The PWV measures the velocity of the central pulse wave and represents a marker of arterial stiffness. Increased PWV is a predictive factor for cardiovascular events, even when considering other established risk factors (Ben-Shlomo et al. 2014). In our study, we were not able to detect any significant effects of the 8-week endurance training intervention on PWV. According to a meta-analysis, aerobic endurance training is generally associated with a significant decrease in the central PWV of − 0.67 m s−1 (Huang et al. 2016). Nevertheless, subgroup analyses in the meta-analysis indicate variations, with reduced effects among healthy individuals (weighted mean difference of − 0.19 m s−1), compared to those with cardiovascular diseases (weighted mean difference of − 0.55 m s−1), longer intervention durations (weighted mean difference 4–8 week interventions: − 0.35 m s−1 vs. > 16 weeks: − 1.19 m s−1), and more significant changes in \(\dot{V}\)O2max/M (weighted mean difference change in \(\dot{V}\)O2max ≤ 10% = − 0.40 m s−1 vs. ≥ 20% = − 1.72 m s−1), and in male compared to female participants (weighted mean difference males: − 0.50 m s−1 vs. females: − 0.36 m s−1) (Huang et al. 2016). The relatively short 8-week intervention duration in our study, coupled with the characteristics of our participants, may explain the limited effect of endurance training on PWV.
Also, no significant changes in the HRV indices could be detected in the INT or CON group. Even though it has been shown that regular endurance training can positively affect HRV parameters, its effectiveness in healthy young to middle-aged individuals is still subject to critical discussion (Dutra et al. 2013). The inclusion of only young and healthy individuals may account for the absence of significant results in the current study. Regarding the individual responses, only 14% of the participants in each group reached the MD.
Limitations
Some limitations should be considered when interpreting the present results.
First, the MC was determined by calendar-based counting, daily basal body temperature and ovulation measurements due to financial limitations. Even though this is a practical and cost-effective approach to determining MC phases, accuracy is limited. This restricts further interpretations regarding hormone changes between responders and non-responders to the training program. Future studies should apply a three-step verification, including serum/plasma hormone analysis, to verify menstrual cycle phases and hormonal concentrations (Johnson et al. 2018; Schaumberg et al. 2017) and investigate the effects of individual hormone levels on the training responsiveness.
Second, we only included healthy participants with a regular MC to reduce the heterogeneity and following ambiguity in the results. However, moderately active, naturally menstruating females represent only part of society, as MC irregularities are highly prevalent among females worldwide (Gimunová et al. 2022; Righi and Barroso 2022). Further, results in a highly trained population where even minor biological differences are important might differ from our study population of moderately active females. This limits the overall generalizability of our results to a broader population, including less or more physically active females or females with menstrual irregularities or disorders.
Third, to ensure a comparable training load between the INT and CON, the training session distribution of two out of three MC phases was manipulated between the two groups, and one MC phase (early to mid-luteal phase) remained constant. Therefore, the overall impact was possibly too low to evoke differences between the MC-adapted and contrary-MC-adapted training, especially over the course of 8 weeks.
Finally, our study consisted of a relatively small sample size due to the exclusion of participants prior to the intervention and before the data analysis, as well as dropouts during the intervention, leading to a decreased statistical power. Based on a post-hoc analysis of our \(\dot{V}\)O2max results, we conducted a required sample size analysis with G*Power 3.1. (Faul et al. 2007) for an ANOVA with repeated measures and within-between interaction. With a given power of 0.8 a total sample size N = 88 participants (n = 44 per group) would be required. Hence, it must be acknowledged that the study may have been underpowered.
However, the current study can contribute to future sample size estimations by providing results for power calculations. Future studies should also consider recruiting larger samples to compensate for possible dropouts for the non-fulfillment of inclusion criteria related to the MC (Elliott-Sale et al. 2021).
Conclusion
In summary, based on our results, we conclude that among healthy, naturally menstruating females, an 8-week polarized running training program consisting of three weekly training sessions significantly enhances performance but has no significant effect on cardiovascular parameters. Furthermore, our study did not reveal any discernible differences in performance and cardiovascular parameters between MC-adapted and contrary-MC-adapted training approaches. This contradicts previous studies on MC-based resistance training reporting positive effects on various performance parameters but aligns with prior research on endurance training. Future studies with an extended intervention period, a larger sample size, and a more reliable MC determination are warranted to advance our understanding of the influence of training adaptation in relation to the MC phase. Based on our findings, it appears that MC-adapted training may not provide discernible performance benefits in moderately trained women. However, this observation does not diminish the importance of considering cycle phases in training planning. The heightened responder rate underscores the significant individual variability in how the MC influences training outcomes and physiological adaptations. Therefore, it is prudent for athletes and trainers to adopt a highly personalized approach when addressing this matter.
In future investigations, several methodological changes and research avenues could be explored. These include alterations in the training intervention, involving variations in training frequency with a focus on high-intensity interval training or moderate intensity continuous training. It could also entail considering other outcomes such as wellbeing and enjoyment, particularly among recreationally active females. Further, investigating whether adapting training to MC phases yields differential effects in elite athletes or among females with MC irregularities could provide valuable insights. Also, we would recommend a prolonged intervention duration, at least three or more MCs.
Data availability
The data presented in this study are available on request from the corresponding author.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CON:
-
Control group
- d :
-
Cohen's d
- HR:
-
Heart rate
- HRV:
-
Heart rate variability
- ICC:
-
Intraclass correlation coefficient
- INT:
-
Intervention group
- MC:
-
Menstrual cycle
- MD:
-
Mean difference to be considered real
- η p 2 :
-
Partial eta squared
- PWV:
-
Pulse wave velocity
- restHR:
-
Resting heart rate
- RMSSD:
-
Root mean square of successive RR interval differences
- RPE:
-
Rate of perceived exertion
- SDNN:
-
Standard deviation of NN intervals
- SEM:
-
Standard error of measurement
- v :
-
Velocity
- \(\dot{V}\)O2max:
-
Maximal oxygen uptake
- VT1:
-
Ventilatory threshold 1
- VT2:
-
Ventilatory threshold 2
- WHtR:
-
Waist-to-height ratio
References
Amann M, Subudhi AW, Walker J, Eisenman P, Shultz B, Foster C (2004) An evaluation of the predictive validity and reliability of ventilatory threshold. Med Sci Sports Exerc 36(10):1716–1722. https://doi.org/10.1249/01.MSS.0000142305.18543.34
Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C-H, Cruickshank JK (2014) Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63(7):636–646
Bentz D, Steiner M, Meinlschmidt G (2012) SIPS-Screening-Instrument für prämenstruelle Symptome: Die deutsche Version des Premenstrual Symptoms Screening Tool zur Erfassung klinisch relevanter Beschwerden. Nervenarzt 83(1):33–39. https://doi.org/10.1007/s00115-010-3210-6
Bernstein C, Behringer M (2023) Mechanisms underlying menstrual cycle effects on exercise performance: a scoping review. Women Sport Phys Act J. https://doi.org/10.1123/wspaj.2022-0026
Boisseau N, Isacco L (2021) Substrate metabolism during exercise: sexual dimorphism and women’s specificities. Eur J Sport Sci. https://doi.org/10.1080/17461391.2021.1943713
Carmichael MA, Thomson RL, Moran LJ, Wycherley TP (2021) The impact of menstrual cycle phase on athletes’ performance: a narrative review. Int J Environ Res Public Health 18(4):1667. https://doi.org/10.3390/ijerph18041667
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sports Med 37(9):737–763. https://doi.org/10.2165/00007256-200737090-00001
Cohen J (2013) Statistical power analysis for the behavioral sciences. In: Statistical power analysis for the behavioral sciences. Routledge. https://doi.org/10.4324/9780203771587
Cornelissen VA, Smart NA (2013) Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2(1):e004473. https://doi.org/10.1161/JAHA.112.004473
Cornelissen VA, Buys R, Smart NA (2013) Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens 31(4):639–648. https://doi.org/10.1097/HJH.0b013e32835ca964
de Jonge XJ, Thompson B, Ahreum HAN (2019) Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc 51(12):2610–2617. https://doi.org/10.1249/MSS.0000000000002073
Decroix L, De Pauw K, Foster C, Meeusen R (2016) Guidelines to classify female subject groups in sport-science research. Int J Sports Physiol Perform 11(2):204–213
Devries MC (2016) Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol 101(2):243–249. https://doi.org/10.1113/EP085369
Dutra SGV, Pereira APM, Tezini GCSV, Mazon JH, Martins-Pinge MC, Souza HCD (2013) Cardiac autonomic modulation is determined by gender and is independent of aerobic physical capacity in healthy subjects. PLoS ONE 8(10):e77092. https://doi.org/10.1371/journal.pone.0077092
Elliott-Sale K, Pitchers G (2019) Considerations for coaches training female athletes. Prof Strength Cond 55(December):19–30
Elliott-Sale KJ, Minahan CL, de Jonge XAKJ, Ackerman KE, Sipilä S, Constantini NW, Lebrun CM, Hackney AC (2021) Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med 51(5):843–861. https://doi.org/10.1007/s40279-021-01435-8
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Ferreira AC, Dias JMC, de Fernandes RM, Sabino GS, dos Anjos MTS, Felício DC (2012) Prevalence and associated risks of injury in amateur street runners from Belo Horizonte, MG. Revista Brasileira De Medicina Do Esporte 18:252–255
Flack JM, Adekola B (2020) Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med 30(3):160–164
Franssen PM, Imholz BP (2010) Evaluation of the mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit 15(4):229–231. https://doi.org/10.1097/MBP.0b013e328339be38
Gaskill SE, Ruby BC, Walker AJ, Sanchez OA, Serfass RC, Leon AS (2001) Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sports Exerc 33(11):1841–1848
Gimunová M, Paulínyová A, Bernaciková M, Paludo AC (2022) The prevalence of menstrual cycle disorders in female athletes from different sports disciplines: a rapid review. Int J Environ Res Public Health 19(21):14243. https://doi.org/10.3390/ijerph192114243
Hackney AC (2021) Menstrual cycle hormonal changes and energy substrate metabolism in exercising women: a perspective. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph181910024
Huang C, Wang J, Deng S, She Q, Wu L (2016) The effects of aerobic endurance exercise on pulse wave velocity and intima media thickness in adults: a systematic review and meta-analysis. Scand J Med Sci Sports 26(5):478–487. https://doi.org/10.1111/sms.12495
Hulton AT, Malone JJ, Campbell IT, MacLaren DPM (2021) The effect of the menstrual cycle and hyperglycaemia on hormonal and metabolic responses during exercise. Eur J Appl Physiol 121(11):2993–3003. https://doi.org/10.1007/s00421-021-04754-w
Isacco L, Boisseau N (2017) Sex hormones and substrate metabolism during endurance exercise. In: Hackney A (ed) Sex hormones, exercise and women: scientific and clinical aspects. Springer, Cham, pp 25–54. https://doi.org/10.1007/978-3-031-21881-1
Johnson S, Marriott L, Zinaman M (2018) Can apps and calendar methods predict ovulation with accuracy? Curr Med Res Opin 34(9):1587–1594. https://doi.org/10.1080/03007995.2018.1475348
Julian R, Hecksteden A, Fullagar HHK, Meyer T (2017) The effects of menstrual cycle phase on physical performance in female soccer players. PLoS ONE 12(3):1–13. https://doi.org/10.1371/journal.pone.0173951
Kissow J, Jacobsen KJ, Gunnarsson TP, Jessen S, Hostrup M (2022) Effects of follicular and luteal phase-based menstrual cycle resistance training on muscle strength and mass. Sports Med 52:1–7
Kolić PV, Sims DT, Hicks K, Thomas L, Morse CI (2021) Physical activity and the menstrual cycle: a mixed-methods study of women’s experiences. Women Sport Phys Act J 29(1):47–58. https://doi.org/10.1123/wspaj.2020-0050
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Kubica C, Ketelhut S, Querciagrossa D, Burger M, Widmer M, Bernhard J, Schneider M, Ries T, Nigg CR (2023) Effects of a training intervention tailored to the menstrual cycle on endurance performance and hemodynamics. J Sports Med Phys Fit. https://doi.org/10.23736/S0022-4707.23.15277-7
Leiva RA, Bouchard TP, Abdullah SH, Ecochard R (2017) Urinary luteinizing hormone tests: which concentration threshold best predicts ovulation? Front Public Health 5(November):1–8. https://doi.org/10.3389/fpubh.2017.00320
McLay RT, Thomson CD, Williams SM, Rehrer NJ (2007) Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int J Sport Nutr Exerc Metab 17(2):189–205. https://doi.org/10.1123/ijsnem.17.2.189
McNulty KL, Elliott-Sale KJ, Dolan E, Swinton PA, Ansdell P, Goodall S, Thomas K, Hicks KM (2020) The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med 50(10):1813–1827. https://doi.org/10.1007/s40279-020-01319-3
Moya-Ramon M, Mateo-March M, Peña-González I, Zabala M, Javaloyes A (2022) Validity and reliability of different smartphones applications to measure HRV during short and ultra-short measurements in elite athletes. Comput Methods Progr Biomed 217:106696
Muñoz I, Seiler S, Bautista J, España J, Larumbe E, Esteve-Lanao J (2014) Does polarized training improve performance in recreational runners? Int J Sports Physiol Perform 9(2):265–272. https://doi.org/10.1123/IJSPP.2012-0350
Oosthuyse T, Bosch AN (2010) The effect of the menstrual cycle on exercise metabolism. Sports Med 40(3):207–227. https://doi.org/10.2165/11317090-000000000-00000
Portney LG, Watkins MP (2009) Foundations of clinical research: applications to practice, vol 892. Pearson/Prentice Hall, Upper Saddle River
Prado RCR, Silveira R, Kilpatrick MW, Pires FO, Asano RY (2021) The effect of menstrual cycle and exercise intensity on psychological and physiological responses in healthy eumenorrheic women. Physiol Behav. https://doi.org/10.1016/j.physbeh.2020.113290
Recacha-Ponce P, Collado-Boira E, Suarez-Alcazar P, Montesinos-Ruiz M, Hernando-Domingo C (2023) Is it necessary to adapt training according to the menstrual cycle? Influence of contraception and physical fitness variables. Life. https://doi.org/10.3390/life13081764
Reimers AK, Knapp G, Reimers CD (2018) Effects of exercise on the resting heart rate: a systematic review and meta-analysis of interventional studies. J Clin Med. https://doi.org/10.3390/jcm7120503
Richardson JTE (2011) Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev 6(2):135–147
Righi I, Barroso R (2022) Do recreationally-trained women of different ages perceive symptoms of the menstrual cycle and adjust their training according to phases? Int J Environ Res Public Health 19(21):13841. https://doi.org/10.3390/ijerph192113841
Schaumberg MA, Jenkins DG, Janse de Jonge XAK, Emmerton LM, Skinner TL (2017) Three-step method for menstrual and oral contraceptive cycle verification. J Sci Med Sport 20(11):965–969. https://doi.org/10.1016/j.jsams.2016.08.013
Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, Girdler SS, Kiesner J, Ditzen B, Eisenlohr-Moul TA (2021) How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 123:104895. https://doi.org/10.1016/j.psyneuen.2020.104895
Thompson B, Almarjawi A, Sculley D, Janse de Jonge X (2020) The effect of the menstrual cycle and oral contraceptives on acute responses and chronic adaptations to resistance training: a systematic review of the literature. Sports Med 50(1):171–185. https://doi.org/10.1007/s40279-019-01219-1
Vigh-Larsen JF, Ørtenblad N, Spriet LL, Overgaard K, Mohr M (2021) Muscle glycogen metabolism and high-intensity exercise performance: a narrative review. Sports Med 51(9):1855–1874
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19(1):231–240
Weisell RC (2002) Body mass index as an indicator of obesity. Asia Pac J Clin Nutr 11:S681–S684
Willett HN, Koltun KJ, Hackney AC (2021) Influence of menstrual cycle estradiol-β-17 fluctuations on energy substrate utilization-oxidation during aerobic, endurance exercise. Int J Environ Res Public Health 18(13):10–15. https://doi.org/10.3390/ijerph18137209
Yoo E-G (2016) Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J Pediatr 59(11):425
Zderic TW, Coggan AR, Ruby BC (2001) Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol 90(2):447–453. https://doi.org/10.1152/jappl.2001.90.2.447
Acknowledgements
We would like to thank all athletes for participating in the study.
Funding
Open access funding provided by University of Bern. This research received no external funding. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: CK; methodology: CK and SK; formal analysis and investigation: CK; writing—original draft preparation: CK and SK; writing—review and editing: CK, SK and CRN; supervision: CRN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
The study was conducted in accordance with the 1964 Declaration of Helsinki, and approved by the Institutional Ethics Committee of the University of Bern (Ethics approval number: Nr. 2022-01-00006).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Communicated by Susan Hopkins.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubica, C., Ketelhut, S. & Nigg, C.R. Polarized running training adapted to versus contrary to the menstrual cycle phases has similar effects on endurance performance and cardiovascular parameters. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05545-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05545-9