Abstract
Purpose
This study aimed to investigate the impact of short-term low energy availability (LEA) on vascular function in young, regularly menstruating women.
Methods
Participants were 19 women, aged 22.9 ± 4.2 years, with body mass index 18–30 kg·m2. They were divided into two groups and completed two conditions in a crossover design: a 3-day control condition (CON) with an energy availability of 45 kcals·kgFFM−1·day−1 and a 3-day LEA condition of 15 kcals·kgFFM−1 day–1. Assessments were conducted during the early follicular phase of the menstrual cycle. Outcome measures included forearm blood flow (FBF), heart rate, blood pressure, arterial stiffness, resting energy expenditure (REE), metabolic blood markers and body composition.
Results
Significant time-by-condition interactions were found for resting FBF (p = .004), REE (p = .042), triiodothyronine (p = .006), β-hydroxybutyrate (p = .002) and body mass (p < .001). Resting FBF was 1.43 ± 1.01 and 1.31 ± 0.61 (arbitrary units) at pre and post, respectively, in LEA and 1.52 ± 0.7 and 1.76 ± 0.57 at pre and post in CON. The LEA condition led to a decrease in triiodothyronine (pre: 1.54 ± 0.28, post: 1.29 ± 0.27 ng ml−1), REE (pre: 1588 ± 165, post: 1487 ± 160 kcals day−1) and body mass (pre: 61.4 ± 7.5, post: 59.6 ± 7.3 kg). Changes in resting FBF were significantly correlated with changes in REE in the LEA condition (r = 0.53; p = 0.02).
Conclusion
Short-term LEA modifies regional blood flow and this might contribute to the observed decreased in REE. Findings emphasize the need for careful management of energy availability in populations at risk of LEA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy availability is the amount of energy that remains once exercise energy expenditure is deducted from energy intake and is considered low when optimal function of all remaining bodily processes cannot be maintained (Adkisson et al. 2010). It is estimated that 45% of female endurance athletes and 23% of recreationally active females exhibit symptoms associated with low energy availability (LEA) (Areta et al. 2021; Dill and Costill 1974). LEA lasting several months can cause menstrual disturbances such as amenorrhea (the cessation of menses for ≥ 90 days) via suppression of the hypothalamic–pituitary–ovarian axis, which is characterized by chronic estrogen deficiency (Forstermann and Munzel 2006; Freedson et al. 1998; Gavin et al. 2009). Exercising women with amenorrhea exhibit reduced resting energy expenditure (REE) commensurate with downregulated physiological function and energy conservation in response to LEA (Gonçalinho et al. 2023). An association between LEA and impaired cardiovascular function exists in this population (Greenfield et al. 1963; Headland et al. 2019; Heikura et al. 2018).
Exercising women with amenorrhea and chronic estrogen deficiency have reduced resting systolic blood pressure, pulse pressure, heart rate, and endothelial-dependent and independent dilatory capacity at the brachial artery (Greenfield et al. 1963). Paradoxically, vascular resistance is elevated despite lower resting blood pressure and resting and peak post-ischemic regional blood flow measured at the calf are reduced (Higashi et al. 2002). Endothelial function is regulated by estrogen receptor signaling (Hill and Davies 2001; Izhar et al. 2000), such that estrogen deficiency likely contributes to the vascular effects observed in exercising women with amenorrhea (Higashi et al. 2002; Junejo et al. 2019). These findings hold importance given endothelial dysfunction is involved in cardiovascular disease progression (Koehler et al. 2016).
LEA ≤ 15 fat-free mass−1 day−1 (kcals kgFFM−1 day−1) can cause acute endocrine perturbations such as suppressed triiodothyronine (T3) and leptin within just 3 days (Kojima et al. 2020) In other contexts, these perturbations have also been associated with impaired endothelial and vascular function in vitro (Logue et al. 2019) and in human participants (Loucks et al. 2011). Estrogen concentrations remain stable during short-term LEA lasting up to 5 days in the low hormone phase of the menstrual cycle (Gavin et al. 2009). This offers a window to investigate estrogen-independent effects of LEA on vascular function, which cannot be elucidated by comparing amenorrheic to eumenorrheic women given the coexistence of estrogen deficiency.
A more holistic understanding of the etiology and temporality of vascular effects of LEA is important for exercise performance, recovery, and health. Consequently, this study aimed to examine the effects of short-term LEA in regularly menstruating young women on resting and peak-ischemic blood flow at the forearm and other secondary outcomes associated with LEA or vascular function, including arterial stiffness, blood pressure, heart rate, REE, T3, body mass and composition. It was hypothesized that LEA would reduce resting and peak forearm blood flow compared to a more optimal level of energy availability, while estrogen concentration remained stable.
Methods
Participants and ethics
Participants were recruited using social media and word-of-mouth and met the following eligibility criteria: female, aged 18–40 years, body mass index 18–30 kg m−2, previous three menstrual cycles lasted 21–35 days, non-smoker, not used hormonal contraception or hormone replacement therapy in the previous 3 months, not vegan, never diagnosed with an eating disorder or medical condition known to impact menstrual function, and not currently dieting. Participants were asked if they regularly performed more than three vigorous or five moderate exercise sessions per week and were excluded if they responded “yes”, to minimize de-training effects during the trials. Twenty-one eligible young women provided informed consent. The study was registered at www.clinicaltrials.gov (NCT04790019) and received ethical approval from Loughborough University Ethics Review Sub-Committee.
Experimental design
Participants completed a preliminary testing phase followed by two experimental trials which were completed during the early follicular phase of different menstrual cycles in a randomized and counterbalanced order. Trials were conducted during consecutive menstrual cycles or with one or two cycles between them. Pseudo-random online software was used to perform block randomization (Sealed Envelope Ltd.).
Preliminary testing included one laboratory visit to confirm eligibility, familiarization with REE methods, and completion of the Low Energy Availability in Females Questionnaire (LEAF-Q) (Melin et al. 2015). For the following 3 days, energy intake was measured using a weighed food diary, analyzed using nutritional software (Nutritics v5.64), and physical activity was monitored using a triaxial accelerometer (ActiGraph wGT3X-BT) worn on the non-dominant hip. Accelerations were collected at a sample rate of 90 Hz and analyzed (ActiLife v6.13.4) for average daily activity energy expenditure (DAEE) and time spent in moderate-to-vigorous physical activity (MVPA) using validated cut-points (Melin et al. 2014).
Experimental trials
Each trial started within 4 days following the self-reported onset of menses and involved 3 consecutive days at a set energy availability, either 45 (CON) or 15 kcals kgFFM−1 day−1 (LEA). Participants were instructed to avoid planned and structured exercise so that energy intake provided by the intervention diets was equal to energy availability. An accelerometer was worn during each trial to check for differences in MVPA which could indicate interference with energy availability manipulations. Omnivorous and vegetarian diets providing 45 kcals kgFFM−1 day−1 composed of 50% carbohydrate, 20% protein, and 30% fat were created for a reference individual (see supplementary file 1). The quantity of each ingredient was scaled to each participants pre-intervention FFM to produce CON diet and then divided by three to produce LEA diet. Omnivores and vegetarians were provided the diet that reflected their habitual practices. Ingredients were weighed to within 1 g (Mettler Toledo PL601-S Electronic Scale). Breakfast consumption was supervised at the laboratory and other meals were packaged. A daily multivitamin and mineral supplement (Vitawell A-Z Multivitamins & Minerals) was taken with breakfast during LEA and adherence to diets was confirmed verbally. Participants were instructed to eat all and only what was provided to them by the research team. Black coffee, black tea, and green tea were permitted to improve adherence (but not from midday prior to test visits) and fluid intake was recorded.
Tests were performed between 07:00 and 09:00 in a fasted state on the first morning of each trial and repeated at the same time on the morning after the last day of each trial. Ambient laboratory temperature, humidity, and pressure were 21.6 ± 0.4 °C, 35.6 ± 3.7%, and 1016 ± 4 mmHg, and there were no significant differences between time points (p > 0.309). Body mass and composition were measured using bioelectrical impedance scales (Seca MBCA 515). Further data were collected using indirect calorimetry, brachial sphygmomanometry, pulse wave analysis, venous occlusion plethysmography, then blood sampling. Participants were instructed to avoid strenuous exercise, alcohol, caffeine from midday prior to test visits, and were asked to record and replicate their diet for the 24 h prior to pre-tests in each trial. Participants were provided the same frozen pizza (https://web.archive.org/web/20220116215841/tesco.com/groceries/en-GB/products/291196322) to eat between 19:00 and 20:00 the evening prior to all pre and post-tests. Frozen pizza quantities provided the evening prior to post-tests (704 ± 45 kcals in CON and 233 ± 13 kcals in LEA) were calculated as part of the intervention diet using the process described previously. For the evening prior to pre-tests, the quantity provided matched that of the final day of the control condition. Participants drank 500 ml of water upon waking on the morning of each test visit and a urine sample of their first void was analyzed for urine specific gravity (USG) using a handheld refractometer. USG and change in plasma volume were considered indicators of hydration status, primarily to aid interpretation of changes in body composition. Whole blood was sampled using the methods described below and used to estimate percentage pre to post-test plasma volume change using the cyanmethemoglobin method (Mendelsohn and Karas 1999). Participants completed the Pittsburgh Sleep Quality Index (PSQI) during pre-tests to produce a cumulative sleep quality score for the previous month.
Indirect calorimetry
REE was assessed via indirect calorimetry in a dark, quiet, temperature-controlled laboratory. Participants lay at rest for 15-min, prior to a mouthpiece and nose clip being fitted, followed by a further 5-min of familiarization with the breathing apparatus. Expired and ambient air were then collected simultaneously in separate Douglas bags for 5-min and used to determine \(\dot{V}\)O2 and \(\dot{V}\)CO2. Percentage O2 and CO2 in expired and ambient samples were analyzed using a gas analysis system (Servomex 1440), calibrated in triplicate on the same day. Total expired volume was measured using a dry gas meter (Harvard Ltd) and standardized to air temperature, ambient pressure, and vapor pressure. The Weir equation was used to calculate REE in kcals day−1. Respiratory exchange ratio (RER) was calculated as \(\dot{V}\)CO2/\(\dot{V}\)O2 for the duration of the gas collection period.
Brachial sphygmomanometry
Participants remained at rest while vascular measures were taken. Resting heart rate plus brachial systolic and diastolic blood pressures were measured in triplicate using a sphygmomanometer (OMRON M7 Intelli IT) and used to calculate mean arterial pressure (MAP). First readings were discarded and an average of the final two was calculated.
Pulse wave analysis
To assess arterial stiffness, pulse wave analysis was performed at the radial artery using a tonometric device and software (SphygmoCor©, AtCor Medical Pty Ltd.). Measures of interest included augmentation index and augmentation index normalized to a HR of 75 bpm (AIx75). Recordings were taken in duplicate, and triplicate if AIx75 differed by > 4%; the average of the closest two was used (Miller and Duckles 2008). Operator index was ≥ 80 for all recordings.
Venous occlusion plethysmography
Resting and peak FBF was measured using venous occlusion plethysmography techniques, see (Mizuma et al. 2001) for a detail review of the method. An appropriately sized 4-wire mercury-in-silastic strain gauge, fitted around the widest part of the forearm, was connected to a plethysmograph (Hokanson EC6, Washington, USA) that had been calibrated using a 2-point volume system: 0 and 1%. A venous occlusion cuff (Hokanson SC10D) attached to a rapid cuff inflation system (Hokanson E20) was placed around the upper arm proximal to the elbow joint and the forearm was positioned slightly above heart level. A wrist cuff was inflated to > 200 mmHg throughout FBF measurements to exclude hand blood flow. The forearm cuff was inflated to 50 mmHg for 7-s (to interrupt venous emptying) and then deflated for 7-s to allow venous emptying. Arterial inflow is proportional to the linear increase in forearm volume during cuff inflation (Morioka et al. 2014). Five successive cuff inflations and deflations were performed. Percentage increase in forearm volume per minute (%FV min−1) over the first three complete and consecutive cardiac cycles following each cuff inflation was analyzed using commercial software (ADInstruments LabChart v8.1.13) and the average was calculated for resting FBF. The same process was then repeated following 5-min of cuff inflation to 200 mmHg, causing forearm ischemia. The first post-ischemic measurement was taken swiftly as possible following deflation from 200 mmHg (i.e., during reactive hyperemia) and %FV min−1 during the first complete cardiac cycle measured was analyzed for peak FBF (Müller et al. 2015). Between-day bias ± 95% limits of agreement for resting and peak FBF was previously estimated in our laboratory as – 0.2 ± 0.5 and -1.2 ± 3.4%FV min−1, respectively, in a sample of 12 young healthy males and females (unpublished data)—comparable to previous research (Napoli et al. 2007). Area under the curve (AUC) during the first complete cardiac cycle was calculated using the trapezoidal method for all five post-ischemic recordings and summed to indicate FBF recovery rate.
Biochemical sampling and analysis
Blood was drawn from an antecubital forearm vein between 09:00–10:00. Samples were collected in K2EDTA and serum separation tubes (BD Vacutainer®, Franklin Lakes, USA) and stored on ice (plasma) or at room temperature (serum) for 30-min before centrifugation at 2,058 G for 15-min at 4 °C. Samples were stored at -80 °C for later analysis. Total T3 and 17β-oestradiol were measured in serum and β-hydroxybutyrate (β-OHB) in plasma. T3 was measured using an automated electro-chemiluminescence immunoassay analyzer (Roche Diagnostics Cobas e411). Inter-assay coefficient of variation (CV) was 3.4%. 17β-oestradiol (intra-assay CVs: 8.3 and 7.8%, inter-assay CV: 8.0%) was measured using a manual enzyme-linked immunosorbent assay as per manufacturers’ instructions (IBL International GmbH). β-OHB (inter-assay CV: 10.2%) was analyzed using an enzymatic spectrophotometric assay (Randox) as per manufacturers’ instructions. All times points for individual subjects were analyzed on the same plate.
Statistical analysis
Data are presented as mean ± standard deviation (SD) unless stated otherwise. Residuals were checked for normality using the Shapiro–Wilk test, cases ≥ 3 SD outside the mean were considered outliers and removed prior to analysis. Non-normal data were transformed prior to analysis using the natural logarithm such that the assumption of normality was not violated. Data are presented as median ± interquartile range for all variables that were transformed prior to analysis. Paired t-test was used to check for differences in measured extraneous variables between CON and LEA. Two-way repeated measures ANOVA was used to investigate time by condition interaction effect for all outcome measures. Partial eta-squared was reported to indicate interaction effect size (ηp2). Pearson correlation was used to identify relationships between variables and all data included in correlations were normally distributed. Data were analyzed using SPSS version 27 (IBM, Chicago, USA) and alpha was set as < 0.05. Cases with missing data were excluded listwise.
Results
Participant characteristics are presented in Table 1. Two participants dropped out after completing one condition: one started using oral contraception and the other re-located. Ambient laboratory temperature, humidity, and pressure were 21.6 ± 0.4°C, 35.6 ± 3.7%, and 1016 ± 4 mmHg, and there were no significant differences between time points (p > 0.309). There was a significant correlation between daily EI and activity exercise expenditure at preliminary testing phase (r = 0.75, p < 0.001).
There were no significant differences in CON vs. LEA for PSQI score (both 4.6 ± 1.0, p > 0.999), MVPA (52 ± 33 vs. 57 ± 30 min, p = 0.438), DAEE (417 ± 213 vs. 439 ± 235 kcals, p = 0.570), daily water intake (2.33 ± 1.10 vs. 2.49 ± 0.92 L, p = 0.230), or pre-post change in plasma volume (– 2.9 ± 4.9 vs. – 0.3 ± 5.0%, p = 0.065).
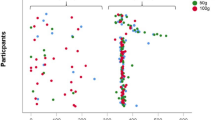
Data and statistical interaction effects regarding body composition, urine specific gravity, metabolic and hormonal markers, and cardiovascular outcomes are presented in Table 2. There were significant time by condition interactions for body mass, FFM, fat-mass, T3, β-OHB, and REE. There was also a significant time by condition interaction for resting FBF (shown in Fig. 1), but not for 17β-oestradiol, peak FBF, FBF AUC during reactive hyperemia, or any other cardiovascular outcome. Pre-post change in the LEA condition for resting FBF was significantly correlated with that of REE (r = 0.52, p = 0.020, see Fig. 2B), but not with that of T3 (r = 0.01, p = 0.968). There was also no significant correlation between REE and FFM (r < 0.01, p = 0.974, see Fig. 2A) or FFM and resting FBF (r = – 0.01, p = 0.971).
Box and whisker plots showing percentage change from pre to post (%) in control (CON) and low energy availability (LEA) conditions for A resting forearm blood flow (FBF), B peak FBF, C AUC during reactive hyperemia (RH), and D AUC during reactive hyperemia relative to peak FBF. Individual data points are shown by circles and crosses shown the mean change. Significant difference compared to CON is indicated (#)
Discussion
This is the first prospective trial on the effect of severe LEA or dietary energy restriction on vascular function in young healthy women. For the first time, we demonstrate that in eumenorrheic non-exercising women assessed during the low-hormone phase of the menstrual cycle, resting FBF is decreased with just 3-days LEA. We also identify an association between resting FBF and REE, which may offer mechanistic insights into metabolic allostasis in response to LEA (Gavin et al. 2009; Gonçalinho et al. 2023).
Resting blood flow
We show that energy availability significantly decreases resting FBF within just 3 days. This may have important health implications for young women. Impaired FBF has been associated with hypertension and increased risk of adverse cardiovascular events (Nishiyasu et al. 1992; O’Donnell et al. 2019; O'Donnell et al. 2014). Long-term LEA can cause chronic estrogen deficiency, with amenorrhea often indicating long-term LEA in active women (Williams et al. 2001; Heikura et al. 2018). Previous research has found that, compared to eumenorrheic counterparts, exercising women with amenorrhea have lower resting calf blood flow (Greenfield et al. 1963), impaired endothelial dependent and independent dilatory function (O'Donnell et al. 2007, 2009), and augmented calf vascular resistance (Headland et al. 2019). Estrogen deficiency, in association with reduced NOS activation and nitric oxide release, is postulated to play a key role (Heikura et al. 2018). However, given our data were collected during the early follicular phase of the menstrual cycle (i.e., the low hormone phase) we suggest that vascular impairments in young premenopausal women may be initiated by LEA, prior to observable reductions in 17β-oestradiol. Future studies may wish to examine whether nitric oxide signaling remains a key factor independent of changes in 17β-oestradiol. Nevertheless, our observed resting FBF was only ~ 26% lower following three days of LEA at 15 versus 45 kcals kgFFM−1 day−1, compared to 48% lower resting calf blood flow on average in amenorrheic athletes versus eumenorrheic counterparts (Greenfield et al. 1963). Differences may be somewhat confounded by measurement site; however, previous research has found little difference in baseline resting blood flows between the two sites (Panaite et al. 2015). It is likely that LEA combined with chronic estrogen deficiency exerts additive adverse effects on regional blood flow in exercising women (Higashi et al. 2002), and that this at least contributes to the differences described, but further investigations are needed.
Resting FBF exhibited a significantly different change during LEA compared to CON; however, post-hoc comparisons in both conditions were not significant. Although measured using different methods, brachial blood flow has been shown to exhibit a natural increase of ~ 26% from early to late follicular phase of the menstrual cycle in young regularly menstruating women (Papageorgiou et al. 2018, 2017), and this might explain the average increase observed during CON. In this context, the interaction effect is an interesting and significant finding given there was no effect on circulating estrogen. The prevention of an increase in resting FBF during LEA was likely linked with other hormonal and metabolic factors associated with short-term energy restriction and vascular function, such as: leptin (Pedrinelli et al. 1995), IGF-1 (Perticone et al. 2001), SIRT-1 (Pinna et al. 2008), or T3 (Qiu et al. 2017). In agreement with previous research, we did not observe a relationship between resting FBF and T3 (Rickenlund et al. 2005); however, T3 infusion has been shown to enhance brachial artery endothelial regulation of vascular tone and resultant blood flow (Qiu et al. 2017). The current study was not designed specifically to look at a relationship between T3 and FBF and a mechanistic role of T3 should not be dismissed based on our findings.
Peak blood flow
In addition to the interaction observed in resting FBF, a similar pattern of change was noted for peak FBF, but the effect of LEA on peak FBF was not statistically significant and there was large interindividual variability. It could be suggested that the study may have been underpowered to detect an effect; however, the conclusion that short-term LEA does not impair peak FBF is consistent with previous research showing that flow mediated dilation (peak dilatory response to augmented flow, indicative of endothelial function) was unaffected by two days of severe energy restriction in healthy men (fed 600 kcals day−1) and women (fed 500 kcals day−1) (Rubanyi et al. 1997). Furthermore, evidence from exercising women with LEA-associated menstrual disturbances such as anovulatory menstrual cycles or amenorrhea lasting ≤ 100 days, suggests that peak calf blood flow may be preserved compared to eumenorrheic counterparts (Higashi et al. 2002). It seems that peak blood flow is more robust to the effects of LEA than resting blood flow. This hypothesis aligns with the life history perspective on LEA, as it would have been an evolutionary advantage to be able to maintain peak blood flow and support intense physical activity (i.e., hunting) during times of food scarcity (Shirley et al. 2022). Nevertheless, peak calf blood flow is significantly lower in exercising women with amenorrhea lasting > 100 days compared to eumenorrheic counterparts (Higashi et al. 2002). Estrogen deficiency elicits transcriptional and translational effects and can cause endothelial dysfunction, accelerated vascular smooth muscle proliferation, and vessel stiffening (Silva et al. 2018). Prolonged exposure to estrogen deficiency may, over time, exert structural limitations to minimum vascular resistance and peak regional blood flow that cannot be overcome.
Our data suggest that short-term LEA does not modify vascular recovery from post-ischemic peak FBF; however, no other data exists in short or longer-term models of LEA by which to compare these findings. Disrupted cardiovascular recovery following different stimuli (such as exercise and psychological stress) has been associated with cardiovascular disease and sudden cardiac death (Steptoe and Marmot 2005; Stoner et al. 2012; Thijssen et al. 2005), and future studies should explore cardiovascular recovery following longer bouts of LEA.
Physiological function and body composition
T3, β-OHB, and glucose all exhibited changes comparable to those of previous research, which have investigated the effects of three to five days LEA in young females, indicating that participants were compliant and LEA was induced (Gavin et al. 2009). This was accompanied by a loss of 1.8 ± 0.6 kg body mass, comparable to 1.8 kg and 1.6 kg lost following three- and five-days LEA, respectively, in similar samples (Kojima et al. 2020; Vecchione et al. 2002). The current data do not indicate any major pre-post differences in hydration status; however, other plausible explanations for a rapid loss of body mass include reduced muscle glycogen and residual gut contents. These data support that participants were not energy deficient upon entry to the study, despite that reported energy intake at the preliminary testing phase seemed lower than total energy expenditure (estimated by summing REE, DAEE, and an additional 10% for dietary induced thermogenesis). However, this is based on group averages and the positive correlation between energy intake and DAEE suggests that those that expended more energy in exercise also had greater energy intakes, and vice versa. Furthermore, average underreporting when using food diaries has been estimated at 18% (Wilkinson and Webb 2001). This equates to ~ 336 kcals in the current study and might also explain the disparity between energy intake and estimated total expenditure during preliminary testing.
Energy restriction has been found to result in physiological adaptations, such as decreased metabolic rate, that reduce daily energy expenditure and the rate of body mass loss (Williams et al. 2015), a phenomenon widely referred to as adaptive thermogenesis (Williams et al. 2001). To the best of our knowledge, we are the first to show a significant reduction in REE (– 100 ± 128 kcals.day−1) under tightly controlled laboratory conditions, during just three days of LEA. RER was > 0.7 and < 1 for all participants at every measurement point. RER reduced from pre to post in LEA and this was likely underpinned by reduced endogenous glycogen stores due to reduced dietary carbohydrate availability (Yoshida et al. 2006). We found no correlation between change in REE and FFM during LEA and suggest that downregulated tissue function (not loss of mass) may be responsible. In support of this, previous research has shown that lower REE in exercise-associated amenorrhea is not due to reduced tissue mass (Gonçalinho et al. 2023). These findings identify that metabolic allostasis can occur rapidly during LEA and underscore the importance of using measures other than changes in body mass to indicate energy status. Furthermore, the positive association between resting FBF and REE raises questions relating to the etiology of metabolic allostasis during LEA. Causality cannot be implied; however, future studies should explore whether endothelial and vascular function are involved in conserving energy during LEA by regulating downstream tissue function or core area kept at body temperature.
Limitations
This study has several strengths, including a robust study design, a valid control condition, detailed measurement of extraneous variables, and the provision of freshly prepared food. There are also some limitations. The sample size is relatively small, but still larger than other studies investigating the effects of LEA utilizing similar methodologies (Gavin et al. 2009). We were unable to estimate an effect size for a priori power calculation given the novelty of this study and analyses should therefore be considered exploratory. Our sample comprised young, regularly menstruating women that were studied exclusively during the early follicular phase and did not perform more than three vigorous or five moderate exercise sessions per week. This limits the generalizability of the findings to other menstrual cycle phases and populations, including athletes. We were not able to identify a specific mechanism for alterations in vascular function observed. Additionally, exercise was avoided during both trials to avoid the known endothelial nitric oxide stimulatory effects of exercise-induced increases in shear stress (Greenfield et al. 1963). As such, vascular responses to LEA induced by exercise expenditure could not be determined in the current study. Although the data suggest otherwise, the reliance on self-adherence to dietary protocols may have compromised internal validity. Future research should consider a more diverse and larger sample size, a wider hormonal and metabolic assessment, concomitant exercise, and stricter methods for dietary adherence.
Conclusion
In conclusion, the data suggest that 3-days of LEA may impact vascular function in young, regularly menstruating women. In addition to the recognized importance of estrogen to vascular health in women, our findings suggest that LEA may independently induce vascular impairments prior to perturbations in estrogen status. This may influence the health and performance of female athletes. Appropriate monitoring of energy availability in the female athlete may help prevent such vascular perturbations. Furthermore, healthcare practitioners should be aware that reduced regional blood flows may be associated with acute or chronic LEA. Further studies to explore the complex relationship between energy availability, hormonal regulation, and cardiovascular health in a broader demographic are warranted.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- %FV min-1:
-
Percentage increase in forearm volume per minute
- AUC:
-
Area under the curve
- AIx75:
-
Augmentation index normalized to a HR of 75 bpm
- β-OHB:
-
β-Hydroxybutyrate
- CON:
-
Control
- DAEE:
-
Daily activity energy expenditure
- FBF:
-
Forearm blood flow
- kcals kgFFM–1 day–1 :
-
Kilocalories per kilogram of fat-free mass per day
- LEA:
-
Low energy availability
- LEAF-Q:
-
Low energy availability in females questionnaire
- MAP:
-
Mean arterial pressure
- MVPA:
-
Moderate-to-vigorous physical activity
- PSQI:
-
Pittsburgh sleep quality index
- REE:
-
Resting energy expenditure
- T3:
-
Triiodothyronine
- USG:
-
Urine specific gravity
References:
Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW (2010) Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood, N.J.) 235:111–118. https://doi.org/10.1258/ebm.2009.009186
Areta JL, Taylor HL, Koehler K (2021) Low energy availability: history, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur J Appl Physiol 121:1–21. https://doi.org/10.1007/s00421-020-04516-0
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248. https://doi.org/10.1152/jappl.1974.37.2.247
Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714. https://doi.org/10.1161/CIRCULATIONAHA.105.602532
Freedson PS, Melanson E, Sirard J (1998) Calibration of the computer science and applications, Inc. Accelerometer. Med Sci Sports Exerc 30:777–781. https://doi.org/10.1097/00005768-199805000-00021
Gavin KM, Seals DR, Silver AE, Moreau KL (2009) Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94:3513–3520. https://doi.org/10.1210/jc.2009-0278
Gonçalinho GHF, Kuwabara KL, Faria NFdO et al (2023) Sirtuin 1 and vascular function in healthy women and men: a randomized clinical trial comparing the effects of energy restriction and resveratrol. Nutrients 15:2949. https://doi.org/10.3390/nu15132949
Greenfield ADM, Whitney RJ, Mowbray JF (1963) Methods for the investigation of peripheral blood flow. Br Med Bull 19:101–109. https://doi.org/10.1093/oxfordjournals.bmb.a070026
Headland ML, Clifton PM, Keogh JB (2019) Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int J Obes 43:2028–2036. https://doi.org/10.1038/s41366-018-0247-2
Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM (2018) Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab 28:403–411. https://doi.org/10.1123/ijsnem.2017-0313
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K (2002) Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 346:1954–1962. https://doi.org/10.1056/NEJMoa013591
Hill RJ, Davies PSW (2001) The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr 85:415–430. https://doi.org/10.1079/BJN2000281
Izhar U, Hasdai D, Richardson DM, Cohen P, Lerman A (2000) Insulin and insulin-like growth factor-I cause vasorelaxation in human vessels in vitro. Coron Artery Dis 11:69–76. https://doi.org/10.1097/00019501-200002000-00012
Junejo RT, Ray CJ, Marshall JM (2019) Cuff inflation time significantly affects blood flow recorded with venous occlusion plethysmography. Eur J Appl Physiol 119:665–674. https://doi.org/10.1007/s00421-018-04056-8
Koehler K, Williams NI, Mallinson RJ, Southmayd EA, Allaway HCM, De Souza MJ (2016) Low resting metabolic rate in exercise-associated amenorrhea is not due to a reduced proportion of highly active metabolic tissue compartments. Am J Physiol Endocrinol Metab 311:E480–E487. https://doi.org/10.1152/ajpendo.00110.2016
Kojima C, Ishibashi A, Tanabe Y et al (2020) Muscle glycogen content during endurance training under low energy availability. Med Sci Sports Exerc 52:187–195. https://doi.org/10.1249/MSS.0000000000002098
Logue DM, Madigan SM, Heinen M, McDonnell S, Delahunt E, Corish CA (2019) Screening for risk of low energy availability in athletic and recreationally active females in Ireland. Eur J Sport Sci 19:112–122. https://doi.org/10.1080/17461391.2018.1526973
Loucks AB, Kiens B, Wright HH (2011) Energy availability in athletes. J Sports Sci 29:S7–S15. https://doi.org/10.1080/02640414.2011.588958
Melin A, Tornberg ÅB, Skouby S et al (2014) The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br J Sports Med 48:540–545. https://doi.org/10.1136/bjsports-2013-093240
Melin A, Tornberg ÅB, Skouby S et al (2015) Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 25:610–622. https://doi.org/10.1111/sms.12261
Mendelsohn ME, Karas RH (1999) The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811. https://doi.org/10.1056/NEJM199906103402306
Miller VM, Duckles SP (2008) Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241. https://doi.org/10.1124/pr.107.08002
Mizuma H, Murakami M, Mori M (2001) Thyroid hormone activation in human vascular smooth muscle cells : expression of type Ii iodothyronine deiodinase. Circ Res 88:313–318. https://doi.org/10.1161/01.RES.88.3.313
Morioka T, Emoto M, Yamazaki Y et al (2014) Leptin is associated with vascular endothelial function in overweight patients with type 2 diabetes. Cardiovasc Diabetol 13:10. https://doi.org/10.1186/1475-2840-13-10
Müller MJ, Enderle J, Pourhassan M et al (2015) Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr 102:807–819. https://doi.org/10.3945/ajcn.115.109173
Napoli R, Guardasole V, Angelini V et al (2007) Acute effects of triiodothyronine on endothelial function in human subjects. J Clin Endocrinol Metab 92:250–254. https://doi.org/10.1210/jc.2006-1552
Nishiyasu T, Shi X, Gillen CM, Mack GW, Nadel ER (1992) Comparison of the forearm and calf blood flow response to thermal stress during dynamic exercise. Med Sci Sports Exerc 24:213–217. https://doi.org/10.1249/00005768-199202000-00009
O’Donnell E, Harvey PJ, Goodman JM, De Souza MJ (2007) Long-term estrogen deficiency lowers regional blood flow, resting systolic blood pressure, and heart rate in exercising premenopausal women. Am J Physiol Endocrinol Metab 292:1401–1409. https://doi.org/10.1152/ajpendo.00547.2006
O’Donnell E, Harvey PJ, De Souza MJ (2009) Relationships between vascular resistance and energy deficiency, nutritional status and oxidative stress in oestrogen deficient physically active women. Clinical Endocrinology (oxford) 70:294–302. https://doi.org/10.1111/j.1365-2265.2008.03332.x
O’Donnell E, Goodman JM, Harvey PJ (2011) Cardiovascular consequences of ovarian disruption: a focus on functional hypothalamic amenorrhea in physically active women. J Clin Endocrinol Metab 96:3638–3648. https://doi.org/10.1210/jc.2011-1223
O’Donnell E, Goodman JM, Mak S, Harvey PJ (2014) Impaired vascular function in physically active premenopausal women with functional hypothalamic amenorrhea is associated with low shear stress and increased vascular tone. J Clin Endocrinol Metab 99:1798–1806. https://doi.org/10.1210/jc.2013-3398
O’Donnell E, Scheid JL, West SL, De Souza MJ (2019) Impaired vascular function in exercising anovulatory premenopausal women is associated with low bone mineral density. Scand J Med Sci Sports 29:544–553. https://doi.org/10.1111/sms.13354
Panaite V, Salomon K, Jin A, Rottenberg J (2015) Cardiovascular recovery from psychological and physiological challenge and risk for adverse cardiovascular outcomes and all-cause mortality. Psychosom Med 77:215–226. https://doi.org/10.1097/PSY.0000000000000171
Papageorgiou M, Elliott-Sale KJ, Parsons A et al (2017) Effects of reduced energy availability on bone metabolism in women and men. Bone (new York, N.y.) 105:191–199. https://doi.org/10.1016/j.bone.2017.08.019
Papageorgiou M, Martin D, Colgan H et al (2018) Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone (new York, N.y.) 114:181–188. https://doi.org/10.1016/j.bone.2018.06.016
Pedrinelli R, Dell’Omo G, Catapano G et al (1995) Relationship between carotid wall thickness and forearm blood flow reserve in hypertension. Coron Artery Dis 6:845–850
Perticone F, Ceravolo R, Pujia A et al (2001) Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104:191–196. https://doi.org/10.1161/01.CIR.104.2.191
Pinna C, Cignarella A, Sanvito P, Pelosi V, Bolego C (2008) Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor α agonists. Hypertension (dallas, Tex. 1979) 51:1210–1217. https://doi.org/10.1161/HYPERTENSIONAHA.107.106807
Qiu S, Cai X, Sun Z et al (2017) Heart Rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studies. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.005505
Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL (2005) Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. J Clin Endocrinol Metab 90:1354–1359. https://doi.org/10.1210/jc.2004-1286
Rubanyi GM, Freay AD, Kauser K et al (1997) Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Investig 99:2429–2437. https://doi.org/10.1172/JCI119426
Shirley MK, Longman DP, Elliott-Sale KJ, Hackney AC, Sale C, Dolan E (2022) A life history perspective on athletes with low energy availability. Sports Med 52:1223–1234. https://doi.org/10.1007/s40279-022-01643-w
Silva AM, Júdice PB, Carraça EV, King N, Teixeira PJ, Sardinha LB (2018) What is the effect of diet and/or exercise interventions on behavioural compensation in non-exercise physical activity and related energy expenditure of free-living adults? A systematic review. Br J Nutr 119:1327–1345. https://doi.org/10.1017/S000711451800096X
Steptoe A, Marmot M (2005) Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens 23:529–536. https://doi.org/10.1097/01.hjh.0000160208.66405.a8
Stoner L, Young JM, Fryer S (2012) Assessments of arterial stiffness and endothelial function using pulse wave analysis. Int J Vasc Med 2012:903107–903109. https://doi.org/10.1155/2012/903107
Thijssen DHJ, Bleeker MWP, Smits P, Hopman MTE (2005) Reproducibility of blood flow and post-occlusive reactive hyperaemia as measured by venous occlusion plethysmography. Clin Sci (1979) 108:151–157. https://doi.org/10.1042/CS20040177
Vecchione C, Maffei A, Lembo G et al (2002) Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes (new York, N.y.) 51:168–173. https://doi.org/10.2337/diabetes.51.1.168
Wilkinson IB, Webb DJ (2001) Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 52:631–646. https://doi.org/10.1046/j.0306-5251.2001.01495.x
Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL (2001) Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86:5184–5193. https://doi.org/10.1210/jcem.86.11.8024
Williams NI, Leidy HJ, Hill BR, Lieberman JL, Legro RS, De Souza MJ (2015) Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am J Physiol Endocrinol Metab 308:E29–E39. https://doi.org/10.1152/ajpendo.00386.2013
Yoshida N, Ikeda H, Sugi K, Imaizumi T (2006) Impaired endothelium-dependent and – independent vasodilation in young female athletes with exercise-associated amenorrhea. Arterioscler Thromb Vasc Biol 26:231–232. https://doi.org/10.1161/01.ATV.0000199102.60747.18
Acknowledgements
This research was supported by the ACSM Foundation Doctoral Student Research Grant from the American College of Sports Medicine Foundation and Loughborough University School of Sport, Exercise and Health Sciences internal research fund.
Funding
This research was supported by the ACSM Foundation Doctoral Student Research Grant (#20–01289) from the American College of Sports Medicine Foundation and Loughborough University School of Sport, Exercise and Health Sciences internal research fund.
Author information
Authors and Affiliations
Contributions
Mark Hutson, Aiden Chauntry, Emma O’Donnell, and Richard Blagrove all contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mark Hutson and Kyle McConnell. The first draft of the manuscript was written by Mark Hutson and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by Klaas R Westerterp.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hutson, M.J., O’Donnell, E., McConnell, K. et al. Energy availability modulates regional blood flow via estrogen-independent pathways in regularly menstruating young women. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05497-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05497-0