Abstract
Physical activity (PA) has positive effects on various health aspects and neuronal functions, including neuronal plasticity. Exceeding a certain exercise frequency and duration has been associated with negative effects. Our study investigated the effects of excessive PA with a marathon run (MA) and regular PA (training and recovery phases) on electrocortical activity, as measured by electroencephalography (EEG). Thirty healthy marathon runners (26 male, 45 ± 9 yrs) were enrolled in the study. Four resting-state 32 channel EEG recordings were conducted: 12–8 weeks before MA (T-1), 14–4 days prior to MA (T0), 1–6 days after (T2), and 13–15 weeks after MA (T3). Power spectrum analyses were conducted using standardized Low-Resolution Electromagnetic Tomography (sLORETA) and included the following frequency bands: delta (1.5–6 Hz), theta (6.5–8.0 Hz), alpha1 (8.5–10 Hz), alpha2 (10.5–12.0 Hz), beta1 (12.5–18.0 Hz), beta2 (18.5–21.0 Hz), beta3 (21.5–30.0 Hz), and total power (1.5-30 Hz). Statistical nonparametric mapping showed reduced power both in the alpha-2 (log-F ratio = − 0.705, threshold log-F ratio = ± 0.685, p < 0.05) and in the delta frequency band (log-F ratio = −0.699, threshold log-F ratio = ± 0.685, p < 0.05) in frontal cortical areas after MA (T2 vs. T0). These effects diminished at long-term follow-up (T3). The results can be interpreted as correlates for subacute neuroplasticity induced by strenuous and prolonged PA. Although previous studies reported an increase in alpha frequency during and directly postexercise, the adverse observation a few days after exercise cessation suggests counterregulatory mechanisms, whose complex origin can be suspected in subcortical circuits, changes in neurotransmitter systems and modulation of affectivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical activity (PA) and aerobic exercise in particular have been repeatedly associated with various health benefits. Especially well documented are positive effects on cardiovascular aspects, such as hypertension (Diaz and Shimbo 2013; Dimeo et al. 2012), coronary heart disease (Crawford and Morris 1958; Sesso et al. 2000), the metabolic system (Ivy 1997; Kirwan et al. 2017), tumor (Delbruck 2012; Frisch et al. 1985) and overall mortality (Schnohr et al. 2015).
There is increasing evidence of the beneficial effects of PA on neurological and psychiatric diseases, such as dementia, depression and schizophrenia (Emrah Duzel et al. 2016; Falkai et al. 2021; Oertel-Knochel et al. 2014; Schuch et al. 2018). As a result, PA has been included in therapeutic regimens in these cases (Malchow et al. 2013), although the mechanisms behind these observations are not yet fully understood. The induction and modulation of neuroplasticity seems to play a key role in these effects. PA increases cortical capillary supply, induces the development of new neurons (Colcombe et al. 2004), causes alterations in cytoarchitecture, increases cellular proliferation, dendritic complexity (Eadie et al. 2005) and the release of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) (Emrah Duzel et al. 2016). BDNF, in turn, has been positively correlated with an increase in hippocampal volume (Erickson et al. 2012; McKee et al. 2014). In this regard, PA leads to an increase and maintenance of hippocampal volume and thus may support the prevention of cognitive decline and memory loss. This modulation of neuroplasticity also leads to increased cortical connectivity and activation, which has been confirmed in functional MRI studies (Colcombe et al. 2004; Voss et al. 2010).
Regarding the representation of these PA-induced neuroplasticity effects in electroencephalography (EEG), consistent findings are lacking. The previous studies have mainly focused on changes in EEG frequencies during PA and a few minutes after cessation of PA, allowing conclusions to be drawn about acute effects but less about subacute or chronic effects. Moreover, the previous studies mainly focused on certain frequency bands, specific cortical areas, and moderate activity or a single short bout of exercise, termed “acute exercise” (Basso and Suzuki 2017). To our knowledge, there are no studies on neurophysiological effects of such strenuous and prolonged PA as a marathon, demonstrated with EEG. Previous findings showed that strenuous exercise leading to dehydration impairs both information processing and memory functions (Tomporowski 2003). Consistent with negative impact on other health domains (e.g., cardiac repolarization, electrolyte status (Scherr et al. 2012)), strenuous exercise might also have similar detrimental effects on neuroplasticity or electrocortical activity.
Because the plasticity is assumed to represent a subacute and chronic process, a certain time interval between PA cessation and recordings is of great importance. Other neuropsychological outcomes, such as cognitive performance, were shown to improve with a latency of 24 h after marathon running as demonstrated in another study of the ReCaP trial (Roeh et al. 2021).
A common acute effect of PA in the EEG appears to be increased activity, or frontal hemispheric asymmetry, in the alpha frequency band (8–12 Hz), which could be interpreted as an indication of relaxation or a change in affect (Crabbe and Dishman 2004). Possible underlying mechanisms included enhanced somatosensory afferents (Krause et al. 1983; Youngstedt et al. 1993 FEB 01:), changes in norepinephrine concentrations (Stock et al. 1996), hypothalamic regulation (Nielsen et al. 2001), and modulation by subcortical regions such as the thalamus (Larson et al. 1998; Lindgren et al. 1999). However, most of these studies were old, and investigated the cortical effects of moderate PA.
This is the first study investigating the electrocortical effects of regular PA (during the preparation for a MA) and intense PA (MA) at four time points before (T-1, T0), after (T2) and during recovery (T3) from MA, with EEG in the source space carried out by sLORETA analyses. Moreover, the present study is not limited to specific frequency bands or cortical areas but provides current source density analyses covering all frequencies in the range 1.5–30 Hz and all cortical areas. Previous studies showed a temporary increase in alpha-power during and directly after PA cessation and a rapid decay of this effect. Owing to the high intensity of PA in our study, we expect to be able to detect the effect even several days after PA cessation (T2). This design allows us to present for the first time the EEG correlates of regular and intensive PA in a longitudinal design.
Methods
Participants and assessments
The participants were extracted from the study population of the ReCaP trial (Running effects on cognition and plasticity), a longitudinal cooperation study between the Clinic for Psychiatry and Psychotherapy at the LMU University of Munich and the Center for Prevention and Sports Medicine at the Technical University of Munich (Roeh et al. 2020).
Thirty healthy marathon runners participated in this longitudinal study. Inclusion criteria were experience in the field of endurance training (at least one completed half-marathon) as well as sufficient German language skills. Exclusion criteria included age < 18 or > 60 years, relevant pre-existing medical conditions, body mass index (BMI) ≥ 30 kg/m2 and consumption of cannabis. Written informed consent was obtained from each subject. All subjects underwent EEG recordings, demographic analysis (age, sex, smoking, handedness, total education, BMI) and the International Physical Activity Questionnaire (IPAQ). The latter is a validated (self-administered) measuring tool for the assessment of PA (Hagströmer et al. 2006), consisting of 27 questions regarding the previous 7 days’ activities according to domains (occupational PA, transportation PA, housework, house maintenance and caring for family, recreation, sport and leisure-time PA; and time spent sitting) (“Scoring protocol for the International Physical Activity Questionnaire (IPAQ)” 2023), and was assessed at the first visit.
Declarations and ethics statement
The study was conducted in compliance with Good Clinical Practice guidelines, the guiding principles of the Declaration of Helsinki 2008 and local laws and regulations. Our study was reviewed and approved by the ethics committees of the Ludwig Maximilian University Munich Ethics committee (approval reference number 17-148) and the Technical University of Munich (approval reference number 218/17 S).
EEG recordings
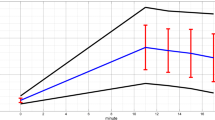
Resting-state EEG was recorded at four different visits around the Munich Marathon 2017, including the training and recovery periods (Fig. 1). The time between the first (T-1) and second (T0) recording represents the intensive training period. T0 represents the tapering phase of MA training where no intensive training took place. No EEG recordings took place on the Marathon day (08.10.2017, T1), as we could not guarantee the same setting of recordings at the marathon site compared to our laboratory, which would result in reduced comparability and we wanted to focus on the subacute and chronic adaptations. The first post-MA EEG recordings (T2) took place 1–6 days and the last recordings (T3) during the resting phase, 13–15 weeks after the marathon.
Resting-state EEG was assessed using electrode caps (“actiCap”, BrainProducts GmbH, Gilching) with 32 electrodes connecting the cap to the control box (“actiCap EEG active electrode system”), which was then connected to an amplifier (“BrainAmp S/N AMP0901983 Standard”). The electrodes were placed on the subject´s head in accordance with the international 10–20 system (Jasper 1958) at FP1, FP2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, TP9, CP5, CP1, CP2, CP6, TP10, P7, P3, Pz, P4, P8, PO9, O1, Oz, O2 and PO10. Electrode skin impedance was less than 25 kΩ, which was achieved by application of a conductivity improving gel (“SuperVisc high-Viscosity Electrolyte-gel For Active Electrodes”, EASYCAP GmbH, Herrsching). Subjects were instructed to be in a resting yet wakeful state while sitting in a comfortably sitting position in a sound and light attenuated room in our laboratory at the university hospital LMU, department of psychiatry with eyes closed, for six-eight minutes. Subjects were supervised during the recording.
Data processing
The EEG data were exported to BrainVision Analyzer Version 2.0 (Brain Products GmbH, Gilching) for further processing. A 70 Hz low-pass filter, a 1 Hz high-pass filter and a 50 Hz notch filter were used, along with a sampling rate of 250 Hz. Prior to data analysis, the artifacts were manually detected and removed (using the Raw Data Inspector function) to exclude eye movements and muscle contractions. From each recording 140 artifact-free EEG segments (one segment = 2000 ms) were used for further analysis and exported to the text ASCII format.
Statistical analyses
Statistical analysis of demographic data
The demographic data (Table 1) were analyzed using SPSS (IBM SPSS Statistics, Version 26). Mean values and standard deviation (SD) were determined using descriptive statistics.
sLORETA analysis
The following three comparisons were performed: T-1 vs. T0 (representing the effects of training for MA), T2 vs. T0 (corresponding to subacute effects of MA) and T3 vs. T0 (illustrating long-term effects of MA and recovery). Current source density analysis was performed in 3-D Talairach/MNI space (Talairach 1988) using the standardized low-resolution electromagnetic tomography (sLORETA, Pascual-Marqui 2002, The KEY Institute for Brain-Mind Research, Zurich, Switzerland). The first version of LORETA (Pascual-Marqui et al. 1994) has been validated broadly using fMRI (Mulert et al. 2004), PET (Zumsteg et al. 2005) and intracerebral recordings (Zumsteg et al. 2006). The version used in our study (sLORETA, (Pascual-Marqui 2002)) is an advanced version of LORETA and estimates the current source density distribution and source localization in 6.239 cortical gray matter voxels, with a cubic voxel size of 5 mm3. For the comparisons across measurements, the sLORETA statistical nonparametric mapping tool (SnPM) was used, based on a paired voxel-by-voxel log-F ratio test using 5.000 randomizations (Villafaina et al. 2019), and included the following frequency bands: delta (1.5–6 Hz), theta (6.5–8.0 Hz), alpha1 (8.5–10 Hz), alpha2 (10.5–12.0 Hz), beta1 (12.5–18.0 Hz), beta2 (18.5–21.0 Hz), beta3 (21.5–30.0 Hz), total power (1.5-30 Hz). Statistical significance levels were set to threshold p < 0.05 and threshold p < 0.10 was considered as a nonsignificant trend-level finding. The null hypothesis of no activation anywhere in the brain was rejected if at least one test value was above the critical threshold for p = 0.05 (Eugene et al. 2015; Horacek et al. 2007). 3D, functional sLORETA images (Figs. 2–4) were calculated representing the electrical current density changes of each voxel in the neuroanatomic Talairach/MNI space (Keeser et al. 2011; Villafaina et al. 2019), corresponding to the estimated neuronal generators of brain activity within each frequency band (Frei et al. 2001). This methodology corrects for multiple testing (i.e., for the collection of tests performed for all electrodes and voxels, and for all time samples. Owing to the nonparametric nature of the method, its validity need not rely on any assumption of Gaussianity (Methods: Statistical analysis of sLORETA / eLORETA 2023; Pascual-Marqui 2002).
Results
Demographics
Of the 30 enrolled subjects that participated in the T-1 visit (26 males, mean age = 44.6 years, SD = 9.5), 7 subjects (5 males) dropped out at various points during the study, due to time constraints, as well as medical issues (internal or orthopedic diseases), resulting in 26 subjects (23 males) whose EEG data were included in at least one of the three EEG comparisons and 23 subjects (21 males) completing all four visits of the study. Descriptive and anthropometric data of all subjects who were included in at least one EEG comparison (N = 26) are shown in Table 1.
EEG results
Effects of marathon training (T-1 vs. T0)
sLORETA nonparametric mapping (SnPM) was applied to determine and localize changes in frequency bands. The T-1 vs. T0 comparison (N = 26) showed no significant differences in delta (log-F ratio = − 0.270), theta (log-F ratio = 0.313), alpha1 (log-F ratio = 0.402), alpha2 (log-F ratio = − 0.447), beta1 (log-F ratio = 0.240), beta2 (log-F ratio = 0.214), beta3 (log-F ratio = 0.318), or total power (log-F ratio = 0. 0.202), (threshold log-F ratio for p < 0.05 = ± 0.682).
Post-marathon vs. pre-marathon (T2 vs. T0)
sLORETA nonparametric mapping (SnPM) was applied to determine and localize changes in frequency bands. The T2 vs. T0 comparison showed reduced alpha-2 (10.5–12.0 Hz) power as well as reduced delta (1.5–6 Hz) power in the post-MA condition (T2) as compared to the pre-MA condition (T0). The maximal difference in alpha-2 power was detected in the right frontal gyrus (N = 24, Brodmann area (BA) 8, MNI coordinates: x = 5, y = 35, z = 55, log-F ratio = − 0.705, threshold log-F ratio = ± 0.685, p < 0.05) (Fig. 2, Table 2). The maximal difference in delta power was detected bilaterally in the frontal gyrus (N = 24, BA 6, MNI coordinates: x = 40, y = 0, z = 50, log-F ratio = − 0.699, threshold log-F ratio = ± 0.685, p < 0.05) (Fig. 3, Table 2). The other frequency bands (theta, alpha1, beta1, beta2, beta3, total power) showed no significant differences.
sLORETA slice viewer, T2 vs. T0: LORETA statistical maps of the alpha-2-frequency band; statistical non-parametric mapping (SnPM) of within-subject comparisons were performed to compare the current density distributions between T2 (T2 = 1–6 days after the marathon) and T0 (T0 = 14 to 4 days prior to the marathon); a significant reduction was detected for the alpha-2 power in T2 when compared to T0, maximum current density differences were localized in the right frontal cortex (xyz = 5, 35, 55; BA 8); the color bar represents the log-F-ratio value for each voxel
sLORETA slice viewer, T2 vs. T0: LORETA statistical maps of the delta-frequency band; statistical non-parametric mapping (SnPM) of within-subject comparisons were performed to compare the current density distributions between T2 (T2 = 1–6 days after the marathon) and T0 (T0 = 14 to 4 days prior to the marathon); a significant reduction was detected for the delta power in T2 when compared to T0, maximum current density differences were localized in the frontal cortex (xyz = 40, 0, 50; BA 6); the color bar represents the log-F-ratio value for each voxel
Long-term follow-up (T3 vs. T0)
The T3 vs. T0 comparison showed no significant differences in electrical cortical activity (delta: log-F ratio = − 0.711, theta: log-F ratio = − 0.747, alpha1: log-F ratio = − 0.824, alpha2: log-F ratio = − 0.761, beta1: log-F ratio = − 0.700, beta2: log-F ratio = − 0.589, beta3: log-F ratio = − 0.597, total power: log-F ratio = − 0.637, threshold log-F ratio for p < 0.05 = ± 0.828). A statistical trend of reduced alpha-1 (8.5–10 Hz) power was detected in the recovery condition (T3) as compared to the pre-MA condition (T0). The maximum current density differences were detected in the left temporal cortex, BA 39, MNI coordinates: x = − 60, y = − 60, z = 25, N = 23, log-F ratio = − 0.824, threshold log-F ratio = ± 0.805, p < 0.10) (Fig. 4, Table 2).
sLORETA slice viewer, T3 vs. T0: LORETA statistical maps of the alpha-1-frequency band; statistical non-parametric mapping (SnPM) of within-subject comparisons were performed to compare the current density distributions between T3 (13 to 15 weeks after the marathon) and T0 (T0 = 14 to 4 days prior to the marathon); no significant frequency changes were detected. A statistical trend was detected in form of reduction in alpha-1 power in T3 when compared to T0, Maximum current density differences were localized in the left temporal cortex, (xyz = − 60, − 60, 25, BA 39); the color bar represents the log-F-ratio value for each voxel
Discussion
In this study we investigated the effects of moderate intensity PA (preparation for a marathon), strenuous and prolonged PA (MA) and subacute and long-term follow-up on the electrocortical activity of healthy adults in four different phases during training for and recovery from MA. We detected a significant reduction in alpha-2 (10.5–12.0 Hz) power as well as a reduction in delta (1.5–6 Hz) power in the post-MA condition (T2) when compared to the pre-MA condition (T0). When we compared the recovery condition (T3) with baseline (T0), no significant differences in EEG frequency bands were found.
J. Crabbe and R. Dishman reported in their quantitative synthesis a frequently observed increased activity, or frontal hemispheric asymmetry, in the alpha frequency band during and shortly after moderate PA (Crabbe and Dishman 2004). The included studies had significant differences in study design (duration, intensity of PA, concrete timing of the EEG). To avoid reduced comparability resulting from unidentical recording settings, EEG recordings were not performed immediately after MA at the marathon site; therefore, we could not confirm or refute this finding. Our post-MA recordings took place 1–6 days after MA (T2), a phase that reflects subacute effects of MA training and completion.
Alpha frequency
There are several possible underlying mechanisms for a subacutely reduced alpha frequency band after PA, some of which can be interpreted as counterregulatory effects following a possibly initially increased alpha frequency, as described by other investigators (Stock et al. 1996; Crabbe and Dishman 2004; Krause et al. 1983; Gutmann et al. 2015; Lindgren et al. 1999; Nielsen et al. 2001; Youngstedt et al. 1993 FEB 01:). Discussed underlying components for a PA-induced increase in alpha frequency included thermoregulatory mechanisms, which in turn play a complex mediating role in mood changes (Crabbe and Dishman 2004; Youngstedt et al. 1993 FEB 01:), neurochemical changes (Crabbe and Dishman 2004; Fumoto et al. 2010), increased somatosensory afferents (Crabbe and Dishman 2004; Krause et al. 1983; Youngstedt et al. 1993 FEB 01:) and a high level of arousal and preparedness for external input (Gutmann et al. 2015).
Some previous studies described this acute frequency increase as indicative of central fatigue (Krause et al. 1983), relaxation (Boutcher et al. 1988) or anxiolysis (Petruzzello and Landers 1994). Some authors even postulated that the preexisting fitness level might determine whether an increase in alpha activity indicated exhaustion or relaxation (Boutcher et al. 1988). These effects can be linked to findings associating increased alpha activity with a high level of somatosensory afferents (SSA) induced by strenuous muscle work (Youngstedt et al. 1993 FEB 01:), thereby suggesting that increased SSA can both lead to exhaustion and relaxation, both traceable through elevated alpha activity. When the intensity of SSA has drastically decreased (T2) after maintaining a high level for a significant period (MA), it is likely that counterregulatory mechanisms counteracting the aforementioned effects take place, resulting in a reduction in the alpha frequency band, decreased central fatigue and subsided anxiolysis. The acute anxiolytic effects of PA have also been linked to hypothalamic regulatory circuits postulating similar underlying mechanisms between thermal application and body movement (thermogenic hypothesis) (Youngstedt et al. 1993 FEB 01:). However, the results in this area have been greatly heterogeneous, which may be a consequence of methodological inconsistencies in the studies (DeBoer et al. 2012). Normalization of body temperature after increased body temperature during MA and associated hypothalamic circuits could play an important role in these subacute effects.
PA-induced improvements in mood and mental wellbeing have numerously been described (Dunn and Jewell 2010; Hartescu et al. 2015; Pelletier et al. 2017; Roeh A, 2020; Wipfli et al. 2008), including reduction in depressive symptoms and increase in positive affects in marathon runners when compared to sedentary controls (Roeh et al. 2020). Neurochemical changes are a major component underlying exercise-induced electrocortical adaptations. Correlations between increased alpha power, mood changes, activation and increased oxygenation in the prefrontal cortex (PFC) and elevated serotonin concentration (5-hydroxytryptamine, 5-HT) after PA have previously been described (Fumoto et al. 2010). Our observed post-MA subacute reduction in alpha-2 power was also detected in the PFC (BA 8), an area that plays a major role in cognitive functions and regulation of emotion (Davidson 2001). Our study did not include measurements of serotonin levels, but PA induces increases in serotonin levels as previously described (Fumoto et al. 2010; Medica et al. 2020). Thus, if elevated alpha power is linked to increased serotonin levels after PA cessation, a secondary reduction in alpha frequency a few days after MA could be associated with a compensatory downregulation of serotonin production. Neurochemical confounders are, however, not limited to serotonin, as links have also been described between high (nor-)epinephrine levels and elevated alpha power (Stock et al. 1996). In addition to localization, the hemispheric asymmetry (right > left) in the effect also indicates a connection to mood alterations. There is evidence linking frontal asymmetry, especially in the alpha frequency band, with the effects of neural circuits of affectivity (e.g., Petruzzello and Landers 1994, Davidson 2001, Coan and Allen 2003). There is also evidence linking mood improvements after PA with changes in functional connectivity between the dorsolateral prefrontal cortex (dlPFC) and the temporal region (TMP), two important cortical structures involved in shaping mood, further supporting the affective component in these mechanisms (Ligeza et al. 2021). Our reported effect may be a correlate to complex changes in functional connectivity, which might be verified in further studies.
Delta frequency
In addition to the reduction in the alpha-frequency band, a reduction in the delta frequency band could be detected post-MA (T2) when compared with T0. Changes in delta activity can often be correlated with neurological pathologies, e.g., various forms of intermittent rhythmic delta activity (IRDA) in epilepsy (temporal IRDA; TIRDA) or encephalopathy (frontal IRDA; FIRDA). There are very limited and inconsistent results on changes in delta activity as an effect of PA (i.e. increase directly after moderate PA in Mechau et al. (Mechau et al. 1998) and Stock et al. (Stock et al. 1996), decrease in Oda et al. (Shiro Oda et al. 1999).
A growing body of evidence proves the effects of PA on cognitive functions such as processing speed, reaction time, memory, attention, planning, and behavioral inhibition (Chang et al. 2012, 2014; Gao et al. 2021; Lambourne and Tomporowski 2010; Tomporowski 2003; Tsai et al. 2014; Weng et al. 2015). One recent study showed improved reaction time in a vigilance task session when a 15-min cycling exercise was inserted compared to the no-intervention session, linking these findings to differences in information processing between the groups and complex changes in functional connectivity induced by PA (Gao et al. 2021). Another recent study detected improvements in EEG slowness (delta waves) as a result of both one session and after six weeks of aerobic exercise while also demonstrating improvements in cognitive functions (using mini-mental state examination (MMSE), Montreal cognitive assessment (MoCA), and trail making test (TMT A and B)), suggesting a link between changes in delta activity and changes in cognition (Amjad et al. 2019). These findings substantiate a link between improvements in cognition, changes in functional connectivity and alterations in delta frequency after PA. Another study reported selected reductions in theta power after PA while also reporting improvements in cognitive functions, proving the heterogeneity in these effects (Devilbiss et al. 2019).
Reductions in the delta frequency band have also been reported as effects of brain stimulation methods, such as transcranial direct current stimulation (tDCS) over the PFC (Keeser et al. 2011; Ulrich Palm et al. 2009). Since tDCS is a plasticity-inducing method and changes in frequency bands could display correlates of this plasticity, the hypothesis arises that running a marathon can induce similar effects and that reduction in delta activity induced by PA and tDCS could be explained by common mechanisms. As tDCS has a positive influence on cognition (Polania et al. 2011) but also may relieve depressive symptoms in depression (Bennabi and Haffen 2018; Fregni et al. 2021; Keeser et al. 2011), this observation contributes to the understanding of EEG measurements in combination with neuroplasticity. These findings could be applied to modifications of cognition as well as in the treatment of depression. Future studies should conduct more precise comparisons between tDCS- and PA-induced EEG effects, which will enable a more precise statement about the similarities of the two interventions.
One limitation of our study is the missing EEG recordings at T1 (directly post-MA). We wanted to measure a naturalistic design with a regular marathon run outside a laboratory to obtain real-world data with better transferability. EEG recordings at the marathon site would not achieve the quality standards of the laboratory recordings (light and sound attenuation, similar recording times between subjects) and would therefore be accompanied by too many confounding variables. Another limitation is the difference in the dates of the EEG recordings at each visit (i.e., 4–14 days prior to MA (T0), 1–6 days after MA (T2) and 13–15 weeks after MA (T3)), with higher variability. This also depicts a naturalistic design but reduces comparability. We aimed at measuring daytime consistent throughout the study period; therefore, we could not measure all participants in one or two days. Our study included only four women, which to some extent originates in the underrepresentation of women in marathon runs. Future studies should try to include more women in similar studies and the effect of gender as a covariate should also be taken into account in the statistical model. Finally, to further substantiate a link between changes in alpha frequency and neurochemical confounders (serotonin, (nor-)epinephrine), future studies in this field should include measurements of blood levels of relevant neurotransmitters and simultaneous EEG recordings around marathon running.
In summary, this study showed for the first time that strenuous and prolonged PA (MA) leads to subacute changes in electrocortical activity, presumably representing alterations in neuroplasticity. Specifically, reduced alpha-2 and delta powers were measured a few days after a MA. Possible underlying mechanisms include subcortical (e.g., hypothalamic) regulatory circuits, hormonal and neurochemical changes, complex modulation of affectivity, and changes in functional connectivity. Moreover, these effects were most profound in frontal cortical regions, which play a major role in affect, cognition and decision making. At long-term follow-up, these effects diminished, suggesting that no long-term persistent neuroplasticity effects can be detected by a marathon run using EEG.
Data availability
The data that support the findings of this study are available upon request from the corresponding author.
References
Amjad I, Toor H, Niazi IK, Afzal H, Jochumsen M, Shafique M, Ahmed T (2019) Therapeutic effects of aerobic exercise on EEG parameters and higher cognitive functions in mild cognitive impairment patients. Int J Neurosci 129(6):551–562. https://doi.org/10.1080/00207454.2018.1551894
Basso JC, Suzuki WA (2017) The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast 2(2):127–152. https://doi.org/10.3233/bpl-160040
Bennabi D, Haffen E (2018) Transcranial direct current stimulation (tDCS): a promising treatment for major depressive disorder. Brain Sci. https://doi.org/10.3390/brainsci8050081
Boutcher SH, Landers DM (1988) The effects of vigorous exercise on anxiety, heart rate, and alpha activity of runners and nonrunners. Psychophysiology 25(6):696–702. https://doi.org/10.1111/j.1469-8986.1988.tb01911.x
Chang YK, Labban JD, Gapin JI, Etnier JL (2012) The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res 1453:87–101. https://doi.org/10.1016/j.brainres.2012.02.068
Chang YK, Tsai CL, Huang CC, Wang CC, Chu IH (2014) Effects of acute resistance exercise on cognition in late middle-aged adults: general or specific cognitive improvement? J Sci Med Sport 17(1):51–55. https://doi.org/10.1016/j.jsams.2013.02.007
Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S (2004) cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 101(9):3316–3321. https://doi.org/10.1073/pnas.0400266101
Coan J, Allen JJB (2003) The state and trait nature of frontal EEG asymmetry in emotion. In: Hugdahl K, Davidson RJ (eds) The asymmetrical brain
Crabbe JB, Dishman RK (2004) Brain electrocortical activity during and after exercise: a quantitative synthesis. Psychophysiology 41(4):563–574. https://doi.org/10.1111/j.1469-8986.2004.00176.x
Morris JN, Crawford MD (1958) Coronary Heart disease and physical activity of work. Br Med J 2(5111):1485
DeBoer LB, Powers MB, Utschig AC, Otto MW, Smits JA (2012) Exploring exercise as an avenue for the treatment of anxiety disorders. Expert Rev Neurother 12(8):1011–1022. https://doi.org/10.1586/ern.12.73
Delbruck H (2012) Physical activity and tumor diseases. Internist (berl) 53(6):688–697. https://doi.org/10.1007/s00108-011-2934-0
Devilbiss DM, Etnoyer-Slaski JL, Dunn E, Dussourd CR, Kothare MV, Martino SJ, Simon AJ (2019) Effects of exercise on EEG activity and standard tools used to assess concussion. J Healthc Eng 2019:4794637. https://doi.org/10.1155/2019/4794637
Diaz KM, Shimbo D (2013) Physical activity and the prevention of hypertension. Curr Hypertens Rep 15(6):659–668. https://doi.org/10.1007/s11906-013-0386-8
Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH (2012) Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension 60(3):653–658. https://doi.org/10.1161/HYPERTENSIONAHA.112.197780
Dunn AL, Jewell JS (2010) The effect of exercise on mental health. Curr Sports Med Rep 9(4):202–207. https://doi.org/10.1249/JSR.0b013e3181e7d9af
Eadie BD, Redila VA, Christie BR (2005) Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol 486(1):39–47. https://doi.org/10.1002/cne.20493
Duzel E, van Prag H, Sendtner M (2016) Can physical exercise in old age improve memory and hippocampal function? Brain 139(3):662–673. https://doi.org/10.1093/brain/awv407
Erickson KI, Miller DL, Roecklein KA (2012) The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. https://doi.org/10.1177/1073858410397054
Eugene AR, Masiak J, Kapica J, Masiak M, Weinshilboum RM (2015) Electrophysiological neuroimaging using sLORETA comparing 22 age matched male and female schizophrenia patients. Hosp Chron 10(2):91–98
Falkai P, Schmitt A, Rosenbeiger CP, Maurus I, Hattenkofer L, Hasan A, Heitkamp M (2021) Aerobic exercise in severe mental illness: requirements from the perspective of sports medicine. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-021-01360-x
Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, Brunoni AR (2021) Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 24(4):256–313. https://doi.org/10.1093/ijnp/pyaa051
Frei E, Gamma A, Pascual-Marqui R, Lehmann D, Hell D, Vollenweider FX (2001) Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA). Hum Brain Mapp 14(3):152–165. https://doi.org/10.1002/hbm.1049
Frisch RE, Wyshak G, Albright NL, Albright TE, Schiff I, Jones KP, Witschi J, Shiang E, Koff E, Marguglio M (1985) Lower prevalence of breast cancer and cancers of the reproductive system among former college athletes compared to non-athletes. Br J Cancer 52:885–891
Fumoto M, Oshima T, Kamiya K, Kikuchi H, Seki Y, Nakatani Y, Yu X, Sekiyama T, Sato-Suzuki I, Arita H (2010) Ventral prefrontal cortex and serotonergic system activation during pedaling exercise induces negative mood improvement and increased alpha band in EEG. Behav Brain Res 213(1):1–9. https://doi.org/10.1016/j.bbr.2010.04.017
Gao L, Zhu L, Hu L, Hu H, Wang S, Bezerianos A, Sun Y (2021) Mid-task physical exercise keeps your mind vigilant: evidences from behavioral performance and EEG functional connectivity. IEEE Trans Neural Syst Rehabil Eng 29:31–40. https://doi.org/10.1109/tnsre.2020.3030106
Gutmann B, Mierau A, Hülsdünker T, Hildebrand C, Przyklenk A, Hollmann W, Strüder HK (2015) Effects of physical exercise on individual resting state EEG alpha peak frequency. Neural Plast 2015:717312. https://doi.org/10.1155/2015/717312
Hagströmer M, Oja P, Sjöström M (2006) The international physical activity questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 9(6):755–762. https://doi.org/10.1079/phn2005898
Hartescu I, Morgan K, Stevinson CD (2015) Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res 24(5):526–534. https://doi.org/10.1111/jsr.12297
Horacek J, Brunovsky M, Novak T, Skrdlantova L, Klirova M, Bubenikova-Valesova V, Hoschl C (2007) Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology 55(3–4):132–142. https://doi.org/10.1159/000106055
Ivy JL (1997) Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med 24(5):321–336
Jasper H (1958) The ten-twenty electrode system of the international federation (Appendix of report of the committee on methods of clinical examination in electroencephalography). Electroencephalogr Clin Neurophysiol 10(2):370–375
Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, Mulert C (2011) Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: a standardized low resolution tomography (sLORETA) study. Neuroimage 55(2):644–657. https://doi.org/10.1016/j.neuroimage.2010.12.004
Kirwan JP, Sacks J, Nieuwoudt S (2017) The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. https://doi.org/10.3949/ccjm.84.s1.03
Krause G, Ullsperger P, Beyer L, Gille HG (1983) Changes in EEG power density spectrum during static muscle work. Eur J Appl Physiol Occup Physiol 51(1):61–66. https://doi.org/10.1007/BF00952538
Lambourne K, Tomporowski P (2010) The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res 1341:12–24. https://doi.org/10.1016/j.brainres.2010.03.091
Larson CL, Davidsom RJ, Abercrombie HC, Ward RT, Schaefer SM, Jackson DC, Holden JE, Perlman SB (1998) Relations between PET-derived measures of thalamic glucose metabolism and EEG alpha power. Psychophysiology 35(2):162–169
Ligeza TS, Nowak I, Maciejczyk M, Szygula Z, Wyczesany M (2021) Acute aerobic exercise enhances cortical connectivity between structures involved in shaping mood and improves self-reported mood: An EEG effective-connectivity study in young male adults. Int J Psychophysiol 162:22–33. https://doi.org/10.1016/j.ijpsycho.2021.01.016
Lindgren KA, Larson LC, Schaefer SM, Abercrombie HC, Ward RT, Oakes TR, Holden JE, Perlman SB, Benca RM, Davidson RJ (1999) Thalamic metabolic rate predicts EEG alpha power in healthy control subjects but not in depressed patients. Biol Psychiatry 45(8):943–952. https://doi.org/10.1016/s0006-3223(98)00350-3
Malchow B, Reich-Erkelenz D, Oertel-Knochel V, Keller K, Hasan A, Schmitt A, Falkai P (2013) The effects of physical exercise in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 263(6):451–467. https://doi.org/10.1007/s00406-013-0423-2
McKee AC, Daneshvar DH, Alvarez VE, Stein TD (2014) The neuropathology of sport. Acta Neuropathol 127(1):29–51. https://doi.org/10.1007/s00401-013-1230-6
Mechau D, Mücke S, Weiss M, Liesen H (1998) Effect of increasing running velocity on electroencephalogram in a field test. Eur J Appl Physiol Occup Physiol 78(4):340–345. https://doi.org/10.1007/s004210050429
Medica P, Giunta R, Bruschetta G, Ferlazzo AM (2020) The Influence of training and simulated race on horse plasma serotonin levels. J Equine Vet Sci. https://doi.org/10.1016/j.jevs.2019.102818
Methods: Statistical analysis of sLORETA / eLORETA (2023). https://www.uzh.ch/keyinst/NewLORETA/Methods/MethodsSnPMloreta.htm
Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Möller HJ, Juckel G, Hegerl U (2004) Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage 22(1):83–94
Nielsen B, Hyldig T, Bidstrup F, González-Alonso J, Christoffersen GR (2001) Brain activity and fatigue during prolonged exercise in the heat. Pflugers Arch 442(1):41–48. https://doi.org/10.1007/s004240100515
Oertel-Knochel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V, Hansel F (2014) Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 264(7):589–604. https://doi.org/10.1007/s00406-014-0485-9
Pascual-Marqui RD, Michel CM, Lehmann D (1994) Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18(1):49–65. https://doi.org/10.1016/0167-8760(84)90014-X
Pascual-Marqui RD (2002) Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24(Suppl D):5–12
Pelletier L, Shanmugasegaram S, Patten SB, Demers A (2017) Self-management of mood and/or anxiety disorders through physical activity/exercise. Health Promot Chronic Dis Prev Can 37(5):149–159. https://doi.org/10.24095/hpcdp.37.5.03
Petruzzello SJ, Landers DM (1994) State anxiety reduction and exercise: does hemispheric activation reflect such changes? Med Sci Sports Exerc 26(8):1028–1035
Polania R, Nitsche MA, Paulus W (2011) Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp 32(8):1236–1249. https://doi.org/10.1002/hbm.21104
Davidson RJ (2001) Toward a biology of personality and emotion. Ann N Y Acad Sci 935:191–207. https://doi.org/10.1111/j.1749-6632.2001.tb03481.x
Roeh A, Bunse T, Lembeck M, Handrack M, Pross B, Schoenfeld J, Scherr J (2020) Running effects on cognition and plasticity (ReCaP): study protocol of a longitudinal examination of multimodal adaptations of marathon running. Res Sports Med 28(2):241–255. https://doi.org/10.1080/15438627.2019.1647205
Roeh A, Schoenfeld J, Raab R, Landes V, Papazova I, Haller B, Scherr J (2021) Effects of marathon running on cognition and retinal vascularization: a longitudinal observational study. Med Sci Sports Exerc 53(10):2207–2214. https://doi.org/10.1249/mss.0000000000002699
Roeh A, Lembeck M, Papazova I, Pross B, Hansbauer M, Schoenfeld J, Haller B, Halle M, Falkai P, Scherr J, Hasan A (2020) Marathon running improves mood and negative affect. J Psychiatr Res 130:254–259. https://doi.org/10.1016/j.jpsychires.2020.08.005
Scherr J, Schuster T, Pressler A, Roeh A, Christle J, Wolfarth B, Halle M (2012) Repolarization perturbation and hypomagnesemia after extreme exercise. Med Sci Sports Exerc 44(9):1637–1643. https://doi.org/10.1249/MSS.0b013e318258aaf4
Schnohr P, O’Keefe JH, Marott JL, Lange P, Jensen GB (2015) Dose of jogging and long-term mortality: the Copenhagen City heart study. J Am Coll Cardiol 65(5):411–419. https://doi.org/10.1016/j.jacc.2014.11.023
Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, Hallgren M, Ponce De Leon A, Dunn AL, Deslandes AC, Fleck MP, Carvalho AF, Stubbs B (2018) Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry 175(7):631–648. https://doi.org/10.1176/appi.ajp.2018.17111194
Scoring protocol for the International Physical Activity Questionnaire (IPAQ) 2023. https://sites.google.com/view/ipaq/score.eference
Sesso HD, Paffenbarger RS, Lee IM (2000) Physical activity and coronary heart disease in men: the harvard alumni health study. Circulation 102(9):975–980
Shiro Oda T, Matsumoto KN, Moriya K (1999) Relaxation effects in humans of underwater exercise of moderate intensity. Eur J Appl Physiol 80:253–259
Stock C, Baum M, Rosskopf P, Schober F, Weiss M, Liesen H (1996) Electroencephalogram activity, catecholamines, and lymphocyte subpopulations after resistance exercise and during regeneration. Europ J Appl Physiol 72:235–241
Talairach JP, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publishers, Inc., New York
Tomporowski PD (2003) Effects of acute bouts of exercise on cognition. Acta Psychol (amst) 112(3):297–324. https://doi.org/10.1016/s0001-6918(02)00134-8
Tsai CL, Wang CH, Pan CY, Chen FC, Huang TH, Chou FY (2014) Executive function and endocrinological responses to acute resistance exercise. Front Behav Neurosci 8:262. https://doi.org/10.3389/fnbeh.2014.00262
Palm U, Keeser D, Schiller C, Fintescu Z, Reisinger E, Baghai TC, Mulert C, Padberg F (2009) Transcranial direct current stimulation in a patient with therapy-resistant major depression. World J Biol Psychiatry 10(4):632–635. https://doi.org/10.1080/15622970802480905
Villafaina S, Sitges C, Collado-Mateo D, Fuentes-García JP, Gusi N (2019) Influence of depressive feelings in the brain processing of women with fibromyalgia: an EEG study. Medicine (baltimore) 98(19):e15564. https://doi.org/10.1097/md.0000000000015564
Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF (2010) Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 48(5):1394–1406. https://doi.org/10.1016/j.neuropsychologia.2010.01.005
Weng TB, Pierce GL, Darling WG, Voss MW (2015) Differential effects of acute exercise on distinct aspects of executive function. Med Sci Sports Exerc 47(7):1460–1469. https://doi.org/10.1249/mss.0000000000000542
Wipfli BM, Rethorst CD, Landers DM (2008) The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. J Sport Exerc Psychol 30(4):392–410. https://doi.org/10.1123/jsep.30.4.392
Youngstedt SD, Dishman RK, Cureton KJ, Peacock LJ (1993) Does body temperature mediate anxiolytic effects of acute exercise? J of Appl Physiol 74(2):825–831
Zumsteg D et al (2005) H2(15)O or 13NH3 PET and electromagnetic tomography (LORETA) during partial status epilepticus. Neurology 65(10):1657–1660
Zumsteg D, Lozano AM, Wennberg RA (2006) Depth electrode recorded cerebral responses with deep brain stimulation of the anterior thalamus for epilepsy. Clin Neurophysiol 117(7):1602–1609. https://doi.org/10.1016/j.clinph.2006.04.008
Acknowledgements
We gratefully acknowledge the participants of this trial and the entire ReCaP study team.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest with regard to the content of this report. AH received paid speakership from Otsuka, Lundbeck, Recordati and Janssen and was a member of the advisory boards of these companies and for Rovi. He is a publisher of the German and WFSBP schizophrenia guidelines.
Ethics approval
The study was conducted in compliance with Good Clinical Practice guidelines, the guiding principles of the Declaration of Helsinki 2008 and local laws and regulations. Our study was reviewed and approved by the ethics committees of the Ludwig-Maximilians University Munich Ethics committee (approval reference number 17–148) and the Technical University Munich (approval reference number 218/17 S). The participants provided written informed consent to participate in this study.
Trial registration
http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00012496.
Additional information
Communicated by Westerterp/Westerblad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moussiopoulou, J., Pross, B., Handrack, M. et al. The influence of marathon running on resting-state EEG activity: a longitudinal observational study. Eur J Appl Physiol 124, 1311–1321 (2024). https://doi.org/10.1007/s00421-023-05356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05356-4