Abstract
We compared the differences in cerebrovascular and cognitive function between 13 aerobic exercise trained, older adults and 13 age-, height- and sex-matched sedentary, untrained controls. We determined whether other measures accounted for differences in cerebrovascular and cognitive function between these groups and examined the associations between these functions. Participants undertook anthropometric, mood, cardiovascular, exercise performance, strength, cerebrovascular, and cognitive measurements, and a blood collection. Transcranial Doppler ultrasonography determined cerebrovascular responsiveness (CVR) to hypercapnia and cognitive stimuli. The trained group had a higher CVR to hypercapnia (80.3 ± 7.2 vs 35.1 ± 6.7%, P < 0.001), CVR to cognitive stimuli (30.1 ± 2.9 vs 17.8 ± 1.4%, P = 0.001) and total composite cognitive score (117 ± 2 vs 98 ± 4, P < 0.001) than the controls. These parameters no longer remained statistically different between the groups following adjustments for covariates. There were positive correlations between the total composite cognitive score and CVR to hypercapnia (r = 0.474, P = 0.014) and CVR to cognitive stimuli (r = 0.685, P < 0.001). We observed a relationship between cerebrovascular and cognitive function in older adults and an interaction between regular lifelong aerobic exercise training and cardiometabolic factors that may directly influence these functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal ageing is accompanied by a decline in fluid cognitive ability (ability to respond to novel situations compared with acquired or crystallised knowledge), which is preceded by reduced cerebrovascular structure and function (Harada et al. 2013; Kennedy and Raz 2015; Bangen et al. 2014; Barnes et al. 2012; Rogers et al. 1985; Ainslie et al. 2008; Salthouse 2012; Bliss et al. 2021). This reduction may be attributed to modifiable risks factors that determine cardiometabolic health, including nutrition and physical activity (Toda 2012; Woods et al. 2011; Bangen et al. 2014; Toth et al. 2017; AIHW 2012; Bliss et al. 2021). As cerebrovascular function and cognition decline during ageing, our ability to perform general tasks associated with daily life decreases (Paterson et al. 2007; Taylor 2014; Bliss et al. 2021; Salthouse 2012). Given that the population is rapidly ageing, and the costs associated with loss of independence and chronic diseases on healthcare systems are rapidly increasing (Taylor 2014; Brown et al. 2017), it is imperative that evidence-based strategies to prevent and reduce the decline in cerebrovascular function and cognition are identified, such as aerobic exercise.
Regular exercise has also been shown to help maintain cerebral perfusion in healthy ageing (Ainslie et al. 2008; Rogers et al. 1990; Bailey et al. 2013), which would be an important, simple and cost-effective treatment. For example, Ainslie et al. (2008) reported that the normal age-related decline in mean cerebral blood flow (CBF) velocity (CBFV) in the middle cerebral artery (MCA) was 0.38–0.45% per year between the ages of 18 and 80 years. However, individuals who regularly engaged in at least 150 min of moderate-vigorous intensity aerobic exercise training for at least 2 years (self-reported data and objectively determined using maximal aerobic exercise capacity measurements) demonstrated a 17% higher CBFV across the lifespan compared to age-matched sedentary counterparts (Ainslie et al. 2008). Nevertheless, a limitation to this study was that it did not measure the effects of regular physical activity and exercise on CVR. CVR is the ability to modify regional blood flow in response to specific physiological (e.g. hypercapnia) or psychological stimuli (Willie et al. 2011; Serrador et al. 2000). It is the rapid increase in CBFV in a conduit cerebral blood vessel which is attributable to dilatation of the microvasculature downstream in response to local chemical changes induced by physiological or psychological stimuli (Willie et al. 2011). CVR is an important measure as the cerebral vasculature is highly responsive to its environment, particularly to the partial pressure of arterial carbon dioxide, and to changes in neuronal metabolism. Further, it is the responsiveness of the cerebrovasculature that maintains both cerebral autoregulation and neurovascular coupling (NVC), and, consequently, cerebral function (Miller et al. 2018; Braz et al. 2017; Toth et al. 2017; Duchemin et al. 2012).
Cross-sectional research that has measured the difference in CVR between aerobically exercise trained and untrained individuals has shown mixed findings. Barnes et al. (2013 Barnes et al. (2013) and Marley et al. (2020) reported that CVR to hypercapnia was positively associated with maximal aerobic exercise fitness and Bailey et al. (2013) reported a positive linear relationship between maximal aerobic exercise fitness (measured objectively using maximal exercise capacity tests), CBFV and CVR. However, others have reported no difference in CVR between aerobically exercise trained and untrained individuals (Braz et al. 2017; Miller et al. 2018) or a lower CVR in elderly Masters athletes compared to untrained controls (Zhu et al. 2013; Thomas et al. 2013). The difference in findings is not clear, but may be due to differences in the technique used for testing CBF (Braz et al. 2017; Joris et al. 2018; Willie et al. 2011), variation in ages and training status (Miller et al. 2018), use of subjective or objective measurements to determine exercise training status and capacity, choice of which cranial or extracranial vessel is used for testing (Braz et al. 2017; Willie et al. 2011) or difference in hypercapnia protocols and the derivatives of CVR (Miller et al. 2018).
Maintaining optimal cerebrovascular function may also delay cognitive decline (Bangen et al. 2014; Toth et al. 2017). Cognitive decline throughout the lifespan is well defined and previous research has reported that middle-aged to older adults who participate in regular physical activity and aerobic exercise training or have undertaken an aerobic exercise training intervention (either self-reported or objectively measured using exercise capacity tests) have greater cognitive capacity than sedentary individuals (Anderson-Hanley et al. 2012; Baker et al. 2010; Bossers et al. 2015; Hoffmann et al. 2016; Lautenschlager et al. 2008; Sobol et al. 2016; Vreugdenhil et al. 2012). However, these studies do not typically measure overall cognitive capacity and focus predominantly on one or few cognitive domains, such as executive function or working memory. The focus on more short-term exercise studies is probably associated with the poor compliance and adherence observed with chronic exercise training (Ngandu et al. 2022; Lam et al. 2015). Therefore, this also reduces the ability to determine the long-term impact of chronic exercise interventions on cognition and cerebrovascular function through both intervention trials and longitudinal studies. This highlights a current knowledge gap between short-term changes and chronic adaptations and whether the ‘gap’ can be closed by implementing exercise training regimes. None of these studies have examined the association between cognition and cerebrovascular function.
Only two cross-sectional studies have measured and associated cognition with cerebrovascular function (Rogers et al. 1990; Brown et al. 2010). These studies demonstrated that older adults with higher aerobic fitness had greater cerebrovascular and cognitive function compared to sedentary individuals. However, CVR to psychological stimuli (NVC) was not tested, nor were any correlation analyses between cerebrovascular and cognitive function performed. Here it would be expected that higher cognitive function would be associated with increased cerebrovascular function, while lower cognitive function would be associated with reduced cerebrovascular function. This is important as it measures complex interactions between neuronal metabolic demands and local haemodynamic changes, thus ensuring that the metabolic demands of the brain are met immediately by the vasculature, within the first few seconds of these metabolic demands increasing (Duchemin et al. 2012; Toth et al. 2017). Further, other potential covariates including body composition, cardiovascular function, biochemistry and educational level that may also explain the differences in cerebrovascular and cognitive function between aerobic exercise trained and untrained sedentary individuals were not accounted for. These important variables may directly impact vascular, cerebrovascular and cognitive functions and may also explain the differences between aerobic exercise trained and sedentary, untrained individuals (Bliss et al. 2021). This is because ageing is associated with reductions in vascular, cerebrovascular and cognitive functions and these potential covariates along with sedentary behaviour exacerbate their decline by essentially promoting low-grade systemic inflammation, oxidative stress and reducing endothelial function (see Bliss et al. (2021) for in depth review of mechanisms) (Bangen et al. 2014; Toth et al. 2017; Toda 2012; Woods et al. 2011).
Accordingly, the aims of our study were threefold. First, to compare the differences in cerebrovascular and cognitive function between older adults that had undertaken regular aerobic exercise training and those that were sedentary and untrained. Second, to determine the association between cerebrovascular and cognitive function in these individuals. Finally, to examine if other measures including body composition, cardiovascular function, biochemistry and educational level would account for the differences between trained and untrained groups. We hypothesised, first, that CVR and cognition would be higher in the trained group compared with the untrained group. Second, cerebrovascular and cognitive function would be correlated, thus reflecting an interdependent relationship between these functions.
Methods
Participants
Thirteen aerobic exercise trained older adults and thirteen age-, height- and sex-matched sedentary, untrained controls participated in the study (Table 1). These participants were recruited from South East Queensland, Australia, which includes metropolitan, regional and rural areas, between October 2019 and March 2021. Trained participants were defined as those who had regular participation in at least 150 min of moderate-vigorous intensity aerobic exercise training per week consistently for at least 2 years and untrained participants as those who were physically inactive for at least the previous 2 years (AIHW 2018). The trained individuals were recruited from various sporting clubs, such as road-runner associations, cycling clubs, swimming clubs, gymnasiums, bushwalker clubs and the general public who participate in aerobic exercise but are not associated with any particular sport or club. This was to ensure that we had a mix of participants who undertook different types of aerobic exercise over the lifespan, as the purpose of the study was not to focus on a single mode or type of aerobic exercise. It should be noted that recruitment and testing were terminated earlier than anticipated due to the impacts of COVID-19 and the restrictions placed on gathering, research and travel by the Queensland and Australian Governments. The exclusion criteria were as follows: aged under 50 years or over 80 years; diagnosed cognitive impairment, current smokers; blood pressure ≥ 160/100 mmHg; prescribed insulin, hormone-replacement therapy, or oral anticoagulants; and a significant history of cardiovascular, neurological, cerebrovascular, kidney, liver disease or cancer. Participants were only included in the study if they were on a stable medication treatment plan for conditions that did not contradict the exclusion criteria such as hypertension, dyslipidaemia, type two diabetes and osteoarthritis. Statin therapy was also included in this study, despite the controversy that prolonged statin therapy may reduce both muscle and cognitive function, as no clear conclusions regarding this have been determined (see (Ruscica et al. 2022) for detailed comparison). The Yale Physical Activity Survey (YPAS) (Dipietro et al. 1993), Lifetime Physical Activity Questionnaire (LPAQ) (Friedenreich et al. 1998), and a customised health and wellbeing screen were initially used to determine whether participants met the study criteria, as well as their exercise training status. The LPAQ assessed the types of and length of participation in various physical activities throughout the lifespan and is reported in Table 1 as consistent years exercise training. The YPAS assessed current physical activity status semi-quantitatively including energy expenditure and duration of exercise and is also reported in Table 1. A higher score in these surveys is considered favourable, with the exclusion of sedentary behaviours, such as the sitting index score. All study procedures were approved by the University of Southern Queensland Research Ethics Committee (H19REA015), which adheres to the Declaration of Helsinki. The study was registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12619001291178). All participants provided written, informed consent prior to participation in the study.
Experimental design
The study utilised a cross-sectional design. Each participant visited the laboratory on two separate occasions, at a similar time of day, separated by a minimum of 24 h and a maximum of 7 days. Participants fasted and abstained from caffeinated stimulants for 1 h before visit 1 and 8–12 h before visit 2. They were also requested to refrain from moderate-vigorous intensity exercise for 24 h before each visit and take their daily supplements and medication after each visit was completed. During visit 1, participants undertook anthropometric, cardiovascular, exercise performance, strength, cerebrovascular and cognitive measurements. At their second visit, participants undertook body composition measurements, blood collection and the Profile of Moods State questionnaire. The Profile of Mood States questionnaire calculated mood disturbance by adding the scores of the negative mood state scales (i.e. anger-hostility, confusion-bewilderment, depression-dejection, fatigue inertia, tension-anxiety) and subtracting the positive mood state scale (i.e. vigour-activity) (Heuchert and McNair 2012). Lower values in the negative mood states and total mood disturbance indicates better mood, while higher values in vigour is associated with positive mood. Between visits, participants were asked to complete a nutritional questionnaire (Automated Self-Administered 24-h Dietary Assessment Tool; National Institute of Health (NIH), Bethesda, MA, USA) to assess energy intake over a typical 24 h period (Pannucci et al. 2018).
Basal cerebral haemodynamics
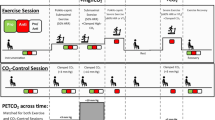
Transcranial Doppler ultrasonography (TCD; DopplerBox X; Compumedics DWL, Singen, Germany) was used to measure basal cerebrovascular haemodynamics, including baseline, peak and mean values for both CBFV and cerebral pulsatility, as well as CVR in response to hypercapnia and cognitive stimuli (Barbour et al. 2017; Evans et al. 2017a; Edmonds Jr et al. 2011). Participants were seated and fitted with a headpiece which housed two 2-MHz TCD ultrasound probes that were fixed and aligned bilaterally to the left and right cranial temporal bone windows to insonate the MCA at a depth of approximately 40–65 mm. Once a suitable blood flow signal was obtained, participants were asked to remain seated for at least 10 min before hypercapnia testing. Following the 10-min period, participants were asked to sit quietly while basal measurements were recorded for 30 s.
Cerebrovascular responsiveness to hypercapnia
Participants were subsequently challenged with a hypercapnic stimulus for 3 min, as a plateau in CBFV is obtained within this time and monitored for another 1 min following removal of this. This process was performed in duplicate following a rest period of at least 5 min (whilst participants breathed in room air in a seated position) to ensure CBFV returned to baseline values (Barbour et al. 2017; Evans et al. 2017a). Participants breathed through a two-way non-rebreathing valve (model 2730, Hans Rudolph, Kansas City, MO, USA) whilst wearing a nose-clip. The inspiratory port of the two-way valve was connected to 1 m of wide bore tubing distal to a 100-L Douglas bag which contained carbogen gas (5% carbon dioxide and 95% oxygen; Carbogen 5; BOC, Toowoomba, QLD, Australia). Flow was measured from the expiratory port of the two-way valve using a pneumotachograph (MLT 300L; AD Instruments, Bella Vista, NSW, Australia) which was calibrated with a 3-L syringe prior to the commencement of each test. Volume was obtained by numerical integration of the flow signal. End-tidal partial pressures of carbon dioxide (PETCO2) were sampled from the expiratory port of the two-way valve connected to a gas analyser (ADI ML206; AD Instruments) that was calibrated across the physiological range with known gas concentrations (BOC). Flow and PETCO2 measurements were sampled at 200 Hz using a 4-channel Powerlab analog-to-digital converter (AD Instruments) interfaced with a computer and displayed in real time during testing. Data were stored for subsequent offline analysis using LabChart software (version 7.2, AD Instruments).
Cognitive function and cerebrovascular responsiveness to cognitive stimuli
The individual cognitive tests that comprised the cognitive battery used to assess neuropsychological function included the Trail Making Task Parts A and B which assessed central executive function, Spatial Span Test (visuospatial short-term working memory) and a NIH Toolbox, which is a battery of cognitive examinations (Evans et al. 2017a; Strauss et al. 2006). The NIH Toolbox is comprised of individuals tests and included the Dimensional Change Card Sort Test (cognitive flexibility and attention); Picture Vocabulary Test (language and crystallised cognition); List Sorting Working Memory Test (working memory); Oral Reading Recognition Test (language and crystallised cognition); Flanker Inhibitory Control and Attention Test (attention and inhibitory control); Picture Sequence Memory Test (episodic memory); and Pattern Comparison Processing Speed Test (processing speed) (Heaton et al. 2014; Slotkin et al. 2012). Additionally, a total composite cognitive function score, which was adjusted and controlled for age, sex, education and ethnicity and derived from all of the tests that comprise both fluid and crystallised measurements indicated above, was determined (Heaton et al. 2014). This is a validated and highly reliable score that represents an overall summation of general cognitive function and indicates general cognitive ability based on normalised scores (Heaton et al. 2014; Weintraub et al. 2014; Slotkin et al. 2012). All tests excluding the Trail Making Task were delivered using an iPad (6th generation, Apple Inc, Cupertino, CA, USA). All NIH Toolbox test scores were automatically computed within the program to control for examiner bias. The outputs for all tests were normalised based on the demographics entered into the program (age, education level, familial education history, sex, ethnicity and occupation). A full description of how these tests are administered, how these scores are calculated and the validation of these tests and scores has been previously described in detail (Heaton et al. 2014; Slotkin et al. 2012; Weintraub et al. 2014). These individual cognitive tests were used for the CVR to cognitive stimuli. The CVR to cognitive stimuli was assessed individually for each cognitive task. Thirty seconds of baseline data were recorded before the start of each cognitive task. The total composite (overall) CVR to all cognitive stimuli was summated and averaged based on the number of tests completed. All participants had the same duration of cognitive stimuli applied – i.e. there were no differences in the cognitive tasks administered to the participants of the study.
Data capture and processing for cerebrovascular responsiveness
Beat-to-beat measurements of CBFV were recorded from the MCA onto software (QL Reader; Compumedics DWL, Singen, Germany) sampling at 100 Hz and were stored for subsequent offline analysis. If a bilateral signal was not obtained, then analysis took place with only the side that was able to be obtained. These data were then normalised and analysed using Curve Expert Professional software (Hyams Development, Chattanooga, TE, USA) to determine resting cerebral pulsatility index (CPI), resting CBFV and peak CBFV. CPI and CVR were calculated based on the equations [1] and [2] from previous work (Harris et al. 2018; Wong et al. 2019; Evans et al. 2017b; Nealon et al. 2017; Wijnhoud et al. 2011)
Anthropometrics and body composition
Participants were instructed to wear light clothing prior to testing and subsequently asked to remove their shoes for measurements. Body mass was measured to the nearest 100 g using an electronic scale (Tanita Ultimate Scale 2000; Tanita, Tokyo, Japan) and waist and hip circumferences recorded to the nearest 1 cm using a standard tape measure as previously described (Welborn et al. 2003). Height was recorded to the nearest 1 cm using a wall-mounted telescopic stadiometer (Seca220; Vogel & Halke, Hamburg, Germany). Height, body mass and waist and hip circumference measurements were measured in duplicate and the mean of the two measurements were analysed. Body mass index (BMI) and a waist-to-hip ratio were calculated as previously described (Welborn et al. 2003; Keys et al. 1972). Dual-energy X-ray absorptiometry was measured to determine body composition of total lean mass, body fat percentage, and whole-body bone mineral content and density (Luna Corp Prodigy Advance Model GE; Madison, WI, USA).
Cardiovascular function
Systolic and diastolic blood pressure, mean arterial pressure and arterial elasticity were measured non-invasively using a HDI/Pulsewave™ CR-2000 Research Cardiovascular Profiling System (Hypertension Diagnostics, Eagan, MN, USA) (Prisant et al. 2002). Participants rested in a seated position for 10 min prior to measurements and four consecutive readings were recorded approximately 5 min apart. An automated oscillometer and an appropriately size blood pressure cuff over the left brachial artery were used to assess blood pressure and a tonometer, placed over the right radial artery, to assess pulse wave analysis, heart rate and estimated cardiac output and cardiac index (Barbour et al. 2017; Prisant et al. 2002). The first reading was discarded, and the mean of the three subsequent measurements were used for analysis.
Biochemical analyses
Approximately 20 ml of venous blood was sampled using a suitable method (either evacuated tube system or winged-infusion) from the veins of the antecubital fossa into thrombin-based clot activator serum separator tubes, 17 IU/ml lithium heparin tubes, 3.2% citrate tubes and 1.8 mg/ml K2 ethylenediaminetetraacetate tubes (BD, Macquarie Park, NSW, Australia). Following collection, blood was either left to stand for 30 min at 18–25 °C (serum separator tube) prior to centrifugation at 1300g and 18 °C for 10 min, or centrifuged immediately at 1300g and 18 °C for 10 min (plasma tubes) as outlined by the tube manufacturer and the testing laboratory (QML 2019; BD 2019). Following centrifugation, blood was either separated as serum or plasma pending the type of tube used to collect the blood. Samples used for the general chemistry profile and high-sensitivity C-reactive protein (hs-CRP) measurements were performed on a Siemens ADVIA® Labcell® (Siemens Healthcare, Bayswater, VIC, Australia), which utilises spectrophotometric (enzymes, metabolites, proteins, lipids), turbidimetric (hs-CRP) and potentiometric (electrolytes) techniques (Healthineers 2019). The remainder of the serum and plasma was stored at -80 °C for subsequent analyses of vascular endothelial growth factor, which was measured in duplicate using an enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Catalogue No. KHG0111; Invitrogen, Human VEGF ELISA Kit, Vienna, Austria).
Exercise performance and handgrip strength
Exercise performance was assessed using a 6-min walk test (6MWT) according to published guidelines (ATS 2002). Handgrip strength was determined using hand dynamometry (Jamar Digital Plus; Lafayette Instruments, Lafayette, IN, USA) with an adequate rest period of 1 min between each attempt to limit fatigue, as previously described (Hillman et al. 2005). Participants were permitted three attempts with both their dominant and non-dominant hands. The first reading for each hand was discarded and was used as a familiarisation and the second and third readings for each hand were averaged for each hand and were used for analysis.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (IBM, Chicago, IL, USA). An initial power calculation was performed on the basis of previous research that has investigated the differences in CVR between population groups (Barbour et al. 2017; Evans et al. 2017a; Wong et al. 2016a, c). The power analysis demonstrated that a sample size of 12 per group would be required to detect a 5% difference in CVR between trained and untrained participants (alpha = 0.05 and power = 0.8), which has been previously shown to be positively correlated with statistically significant improvements in cognition (Barbour et al. 2017). Normality of data was assessed using a Shapiro–Wilk test. Comparisons between groups for anthropometric, body composition, cardiovascular, cognitive, exercise performance, baseline cerebrovascular, baseline respiratory, both CVR to hypercapnia and CVR to cognitive stimuli, strength and biochemical measures were determined using independent t test or Mann–Whitney U-tests for parametric and non-parametric data, respectively. Between-group differences for raw cerebrovascular (excluding CVR) and respiratory measures were analysed using a two-way analysis of variance to determine the effects of ‘group’ (trained vs. untrained) and ‘time’ (baseline vs. peak during hypercapnia). Significant group × time interaction effects were followed by planned pairwise comparisons between groups using the Bonferroni method. Pearson’s product moment correlation coefficient (parametric data) or Spearman’s (non-parametric data) correlation analysis was used to examine the relationship between variables and reported cut-off points to examine these relationships were applied as previously described (Schober et al. 2018). An analysis of co-variance (ANCOVA) was performed using objective (non-self-reported) measures that demonstrated a significant relationship with the primary outcomes (covariates) as independent variables and the primary outcomes (CVR to hypercapnia; CVR to cognitive stimuli; total composite cognitive score) as dependent variables. These objective measures included years of education, 6MWT distance, large arterial compliance, serum hs-CRP concentration, waist circumference, total body fat percentage, heart rate and breathing frequency. Additionally, one measure of total body composition (total body fat percentage) and only biochemical measurements that were considered clinically significant (i.e. were outside reference ranges) were used in the ANCOVA if significant in the correlation analyses. Statistical significance was set at P < 0.05. Data are presented as means ± SEM.
Results
Participant characteristics
Participant characteristics are shown in Table 1. The types of aerobic exercise types that participants undertook, including duration in years, are shown in Supplemental Table 1.There were no differences between the groups for age, sex and height. The trained group had spent more time in education compared to the untrained group. The trained group had a lower body mass, hip circumference, waist circumference, total body fat and hip-to-waist ratio than the untrained group. The trained group walked for longer during the 6MWT and had a higher total energy output, sitting index score and vigorous activity and leisurely walking index score than the untrained group. The trained group had a higher vigour score than the untrained group. There were no other differences in participant characteristics between the groups.
Biochemical analyses
Participants’ general biochemistry profiles, hs-CRP and vascular endothelial growth factor are shown in Table 2. Serum sodium and high-density lipoprotein concentrations were higher in the trained compared to the untrained group. Serum glucose, triglycerides, hs-CRP, alkaline phosphatase and alanine aminotransferase concentrations were lower in the trained compared to the untrained group. There were no other differences in the general chemistry profile and vascular endothelial growth factor between the groups.
Cardiovascular function
Cardiovascular function is shown in Table 3. Heart rate, cardiac output, and systolic, diastolic and mean arterial blood pressure were lower in the trained group compared to the untrained group. Large arterial compliance was higher in the trained group compared to the untrained group. There were no other differences in cardiovascular function between the groups.
Cerebrovascular responsiveness to hypercapnia
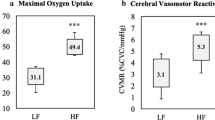
The CVR to hypercapnia is shown in Fig. 1 and Table 4. There were no differences in TCD signal laterality between the groups (untrained unilateral signal, n = 4 vs. trained unilateral signal, n = 5; P = 0.695). There were no differences in CBFV at baseline. The CVR to hypercapnia (53% higher) and CPI were higher for the trained than the untrained group. There was an increase (main effect of time) in CBFV, CPI, PETCO2 and tidal volume in response to hypercapnia. The responses (time × group interaction) of CBFV and CPI were also higher for the trained than the untrained group. Breathing frequency was lower for the trained compared to the untrained group, but there were no time × group interactions.
Cognitive function and cerebrovascular responsiveness to cognitive stimuli
Cognitive function and cerebrovascular responses to cognitive stimuli are shown in Fig. 1 and Table 5. The trained group had higher overall cognitive function than the untrained group, which was demonstrated by a higher total cognitive composite score (17% higher). The trained group also had higher cognitive function than the untrained group in the Dimensional Change Card Sort Test (cognitive flexibility and attention), the List Sorting Working Memory Test (working memory), and Oral Reading Recognition Test (crystallised cognition). The trained group completed both sections of the Trail Making Task (executive function) in less time with fewer errors made in Part B of the task than the untrained group. The time difference between Parts B and A of the Trail Making Task was lower in the trained compared to the untrained group.
The total composite (overall) CVR to all cognitive stimuli was higher in the trained compared to the untrained group (40% higher). The trained group had a higher CVR to cognitive stimuli compared with the untrained groups during the Dimensional Change Card Sort test, Picture Vocabulary Test, Flanker Inhibitory Control and Attention Test, Picture Sequence Memory Test, List Sorting Working Memory Test, Part A of the Trail Making Task and the Spatial Span Test.
Participants were requested to indicate their perceived level of stress and mental fatigue using a digitised visual analogue scale (Visual Scale; Bit Genoma Digital Solutions SL, Badalona, Spain) pre and post cognitive testing.
Correlations between measured variables and cerebrovascular responsiveness to hypercapnia and cognitive stimuli, and cognitive function
Significant correlations between measured variables and CVR to hypercapnia and cognitive stimuli, and cognitive function are shown in Table 6 and Fig. 2. There were moderate positive correlations between CVR to hypercapnia and CVR to cognitive stimuli and the total composite cognitive score. There was a strong positive correlation between the total composite CVR to cognitive stimuli and total composite cognitive score. Both CVR to cognitive stimuli and total composite cognitive score were also strongly correlated with consistent exercise training (years).
There were moderate positive correlations between CVR to hypercapnia and the total years educated, 6MWT distance and large arterial compliance. There were moderate negative correlations between CVR to hypercapnia and serum hs-CRP concentration, waist circumference, total body fat percentage, heart rate and baseline breathing frequency.
There were moderate positive correlations between the total composite cognitive score and total years educated and, 6MWT distance. There were moderate negative correlations between the total composite cognitive score and peak breathing frequency during hypercapnia, waist circumference and heart rate. There was a moderate negative correlation between the total composite cognitive score and large arterial compliance.
There were moderate positive correlations between the total composite CVR to cognitive stimuli and total years educated and 6MWT distance. There were moderate negative correlations between the total composite CVR to cognitive stimuli and waist circumference, heart rate, and breathing frequency both at baseline and peak breathing frequency during hypercapnia.
Analysis of covariance between the primary outcomes and covariates
Objective measures that demonstrated a significant relationship with the primary outcomes (CVR to hypercapnia; CVR to cognitive stimuli; total composite cognitive score) and were considered clinically significant were used in the ANCOVA (described above; shown in Table 6). The reference ranges used for determining clinical significance of these values were derived from the Royal College of Pathologists of Australasia, Royal Australian College of General Practitioners, Australian Heart Foundation and the American Thoracic Society (ATS 2002; Harris 2013; RCPA 2021). Those that demonstrated significant relationships with the primary outcomes and were clinically significant included years of education, 6MWT distance, large arterial compliance, serum hs-CRP concentration, waist circumference, total body fat percentage, heart rate and breathing frequency. Following adjustment for covariates (years of education, 6MWT distance, large arterial compliance, serum hs-CRP concentration, waist circumference, total body fat percentage, heart rate and resting breathing frequency) the ANCOVA revealed that the CVR to hypercapnia no longer remained statistically different between the groups (P = 0.934). The ANCOVA performed for the composite CVR to cognitive stimuli (covariates: total years educated, 6MWT distance, maximum breathing frequency during hypercapnia and at rest, waist circumference measurement and heart rate; P = 0.343) was also not statistically different between the groups. This was the same for the ANCOVA performed for the total composite cognitive score (covariates: total years educated, total composite CVR to cognitive stimuli, 6MWT distance, CVR to hypercapnia, maximum breathing frequency during hypercapnia, waist circumference measurement, heart rate and large arterial compliance; P = 0.202). Education level was also strongly correlated with the primary outcomes. When education was removed from the ANCOVA and all other significant variables listed above were left in the analysis, the results were still insignificant (CVR to hypercapnia, P = 0.246; CVR to total composite of cognitive stimuli, P = 0.193; and total composite cognitive score, P = 0.940).
Discussion
Main findings
The aims of our study were threefold. First, to compare the differences in cerebrovascular and cognitive function between older adults that had undertaken regular aerobic exercise training over the last 40 years and those that were sedentary and untrained. Second, to determine the association between cerebrovascular and cognitive function in these individuals. Finally, to examine if other measures including body composition, cardiovascular function, biochemistry and educational level would account for the differences between trained and untrained groups. In support of our aims, the main findings were that, first, the trained group had undertaken 40 years of aerobic exercise training and had higher cerebrovascular and cognitive functions than the untrained group. Second, there were moderate positive correlations between CVR to hypercapnia and total composite cognitive score and CVR to cognitive stimuli. Finally, following adjustment for covariates, CVR to hypercapnia, total composite cognitive score and CVR to cognitive stimuli were not significantly different between the groups. This suggests that there are many factors that affect cognition and cerebrovascular function that are associated with physical inactivity, such as increased adiposity, blood pressure and heart rate, and that lifelong aerobic exercise training may mitigate these adverse effects.
Cerebrovascular responsiveness to hypercapnia
We observed that the CVR to hypercapnia was higher for the trained than the untrained group. This finding supports previous studies that have reported a higher CVR to hypercapnia in older aerobic trained individuals (Bailey et al. 2013; Barnes et al. 2013; Marley et al. 2020; Rogers et al. 1990; Brown et al. 2010), but not others that have reported no differences between trained and untrained groups (Braz et al. 2017; Thomas et al. 2013; Zhu et al. 2013; Miller et al. 2018). We found no differences in resting CBFV between the trained and untrained groups, which contradicted previous studies that have reported a higher CBFV in aerobic exercise trained individuals (Ainslie et al. 2008; Bailey et al. 2013). However, previous research has also reported no differences in resting CBFV following an exercise intervention or between trained and untrained individuals, respectively (Vicente-Campos et al. 2012; Thomas et al. 2013; Braz et al. 2017). The discrepancy between our findings and others is not clear but may be due to the potential variation in aerobic fitness levels between the trained and untrained participants, the types of training others had participated in and using only a single sex for analysis. For example, Ainslie et al. (2008) only recruited men who participated in cycling or running and did not report the final maximum oxygen consumption test. However, Thomas et al. (2013) only recruited competitive and nationally ranked men and women Masters runners whose maximum oxygen consumption was 41 mL/kg/min compared with sedentary individuals who reported a value of 23 mL/kg/min. Braz et al. (2017) recruited men who participated in any type of aerobic exercise as long as they met the definition of being aerobic exercise trained, but reported similar maximum oxygen consumption test values to Thomas in the trained group (40 mL/kg/min), but much higher values in the untrained group (31 mL/kg/min). Additionally, we also observed a higher peak CBFV in the trained compared to the untrained group and a lower CPI both at rest and during hypercapnia, which is consistent with the literature (Bailey et al. 2013; Marley et al. 2020; Barnes et al. 2013; Mohammadi et al. 2021).
The higher CVR to hypercapnia reflects an enhanced ability of the cerebrovasculature to modify regional blood flow in response to local chemical changes (i.e. carbon dioxide) and maintain cerebral autoregulation. The finding may suggest that regular aerobic exercise training can improve the vasomotor reactivity of the blood vessels and reduce arterial stiffness within the cerebrovasculature (Rogers et al. 1985; Miller et al. 2018). Furthermore, aerobic exercise training could be related to favourable changes in cardiovascular structure and function systemically and this is transferrable centrally (Bailey et al. 2013). For example, it was observed that the trained group had lower central adiposity, blood triglycerides and blood glucose, as well as higher blood high-density lipoprotein concentrations and cardiovascular parameters. Additionally, hs-CRP, which is a marker of chronic low-grade systemic inflammation and predictor of risk of an acute cardiovascular or cerebrovascular event (Jialal and Devaraj 2001), was lower in the trained group. The differences in these parameters between the groups are consistent with the benefits associated with consistent aerobic exercise training (Rossman et al. 2018; Lin et al. 2015). These results also highlight the poor cardiometabolic status of the untrained group and this may place them at greater risk of suffering from both decreased cerebrovascular function and cognitive decline (Toth et al. 2017; Woods et al. 2011; Bliss et al. 2021). The higher concentrations of hs-CRP and metabolic markers, such as blood glucose and triglycerides, in the untrained group may also be an indication of reduced endothelial function, which was observed in this study, thereby promoting increased arterial stiffness, thus reducing blood flow and vasoreactivity (Toth et al. 2017; Woods et al. 2011; Bangen et al. 2014; Toda 2012). The reduction in cerebrovascular function, in turn, reduces the metabolic capacity of the brain, as the brain is no longer supplied as efficiently as it once was with essential nutrients, oxygen and its metabolic waste is no longer removed as quickly as in earlier life (Toth et al. 2017; Bliss et al. 2021; Bangen et al. 2014). Therefore, our results support the notion that aerobic exercise training may modulate these changes, preventing or slowing down these processes that are also associated with ageing.
Cognitive function and cerebrovascular responsiveness to cognitive stimuli
We observed that the total composite cognitive score was higher in the trained compared to the untrained group. While we also evaluated specific cognitive measurements that utilise different cognitive domains, the total composite score evaluates a variety of cognitive domains that are used during activities of daily living (Harvey 2019). Each cognitive domain and subtype can directly impact one another (i.e. they are interdependent) (Harvey 2019; Salthouse 2012). Diseases associated with cognitive decline and neurodegeneration typically do not affect a single domain either, rather they impact all cognitive domains (Harvey 2019; Salthouse 2012). The differences in cognitive function between the groups support the current literature, which indicates that regular aerobic exercise training improves cognitive function (Anderson-Hanley et al. 2012; Baker et al. 2010; Bossers et al. 2015; Hoffmann et al. 2016; Lautenschlager et al. 2008; Sobol et al. 2016; Vreugdenhil et al. 2012). The mechanisms for how aerobic exercise training improves cognitive function in humans is poorly understood. Existing knowledge on this mechanism has been mainly drawn from animals studies (Bliss et al. 2021). Improvements in cognition may be attributed to increased brain-derived neurotrophic factor (BDNF) concentrations both systemically and centrally. BDNF acts to promotes synaptogenesis and has been associated with increased hippocampal volume and spatial memory (Erickson et al. 2011) in humans and improved cognition in animal studies (Vaynman et al. 2004). However, the exact role of BDNF in improving neuroplasticity and cognition in different disease states, such as Parkinson’s disease, still remains inconclusive (Johansson et al. 2020).
We also observed that the total composite CVR to cognitive stimuli, which is referred to as NVC, was higher in the trained compared with the untrained group. NVC is the complex interaction between neuronal metabolic demands and local haemodynamic changes, which ensures that metabolic demands are met by the vasculature (Duchemin et al. 2012; Toth et al. 2017). The mechanisms that lead to the regulation of CBF, which allow for the prevention of both ischaemia and hyperaemia at any time, are extremely complex and yet to be fully elucidated. In any case, it is known that the upregulation of endothelial nitric oxide synthase and the subsequent synthesis and release of nitric oxide from the endothelium is a fundamental component involved in both cerebrovascular autoregulation and NVC (Duchemin et al. 2012; Toth et al. 2017). Only one other study that has measured NVC in older trained and untrained individuals. Fabiani et al. (2014) reported that NVC varied according to aerobic fitness level and that individuals with greater aerobic fitness had a higher NVC capacity. However, the authors of this study did not measure the CVR to cognitive stimuli, rather to visual stimuli. Hence, our study is the first study to specifically measure the CVR to individual cognitive tasks, as well as cumulatively. Further, our study is the first to determine an association between cerebrovascular and cognitive function in this particular cohort. The authors of this study suggested that the difference in the NVC responses between trained and untrained individuals were most probably associated with the cardiovascular system and noted that older participants with low aerobic fitness were also hypertensive and overweight or obese. This is similar to our findings in that the untrained participants met the clinical criteria for obesity as defined by BMI and waist circumference (Harris 2013), which was validated by observing a significantly increased total body fat percentage using dual-energy X-ray absorptiometry. We also observed differences between the groups in markers of cardiometabolic health (i.e. body composition, cardiovascular function and biochemical analyses, such as hs-CRP, glucose and lipid profile). Since ageing and cardiometabolic health both directly impact endothelial function, promote arterial stiffness and chronic low-grade systemic inflammation, it would be expected that anything that favours the senescent phenotype or exacerbates endothelial dysfunction, such as obesity, would directly impact NVC and, in turn, cognition.
Correlations between cerebrovascular and cognitive function
The potential association between cerebrovascular function cognitive function is supported by our finding that total composite cognitive scores were moderately positively correlated with CVR to hypercapnia and strongly positively correlated with CVR to cognitive stimuli. Correlation studies between cerebrovascular function and cognition in trained individuals have only been reported once, in which cerebrovascular function was shown to be a positive predictor of overall cognition in older women (Brown et al. 2010). However, other studies have also reported a correlation between cerebrovascular and cognitive function in healthy post-menopausal women (Wong et al. 2016b), hypertensive older adults (Hajjar et al. 2014), Alzheimer’s disease (Richiardi et al. 2015), and healthy older adults (Keage et al. 2015). It is likely that hypertensive older adults and those with Alzheimer’s disease are sedentary. Therefore, it is difficult to make a conclusion based on our findings on the regular aerobic training-cerebrovascular function-cognition relationship. Further studies will need to delineate these relationships by incorporating exercise status into their studies.
The association between cognitive performance and CVR to hypercapnia may be modest as the latter measures a generalised response of the cerebrovasculature to a chemical stimulus and reflects the ability of the cerebrovasculature to respond solely to this stimulus (e.g. increased concentrations of carbon dioxide) (Barnes et al. 2013; Bailey et al. 2013). This is in contrast to NVC, which describes the mechanical response of the neurovascular unit during localised neuronal activation and exertion (i.e. response to increased neuronal metabolism and signalling) (Zlokovic 2011). Hence, while both parameters may be important to holistically determine cerebrovascular function, it is NVC that is most probably specifically related to cognitive function as potentially indicated by the results of this study (Bliss et al. 2021; Toth et al. 2017; Zlokovic 2011). Further, decreased endothelial function and reduced NVC affect cognition, simply because if the haemodynamic response is blunted then there is insufficient essential nutrients and oxygen being made available to supply neurons during times of increased metabolism (Bliss et al. 2021). This essentially affects the ability of the neurons to perform work efficiently and effectively, thus contributing to decreased cognitive function, which, over time, dissipates further leading to cognitive decline.
Analysis of covariance between the primary outcomes and covariates
When we adjusted for other covariates including body composition, cardiovascular function, biochemistry and educational level that may also explain the differences in cerebrovascular and cognitive function between the trained and untrained groups, the difference between them became statistically insignificant. This finding may suggest that exercise probably modifies multiple physiological variables which have an impact on and interaction with cognitive and cerebrovascular function. It also potentially suggests that having a favourable cardiometabolic profile, which is imperative to maintaining endothelial function, may assist in maintaining the autoregulatory function of the cerebrovasculature throughout the lifespan. The finding may also indicate that exercise could positively contribute to this status by reducing central adiposity, reducing low-grade chronic systemic inflammation, improving cardiovascular structure and function and thereby potentially improving cerebrovascular function and cognition. However, no long-term follow-up studies have been performed to confirm whether improvement in cerebrovascular function will directly improve cognition, but based on empirical studies, the relationship appears to be quite clear (Bliss et al. 2021).
In support of our findings are the results of our correlation analyses. Some of the strongest associations made between our primary outcomes (CVR to hypercapnia, CVR to cognitive stimuli and cognitive function) were with exercise (years consistently performed exercise training and 6MWT distance). Other associations were made between cardiorespiratory variables, central adiposity and low-grade systemic inflammation. Aerobic exercise training reduces adiposity and the chronic low-grade systemic inflammation that is associated with increased central adiposity (You et al. 2013; Keating et al. 2015). Additionally, reductions in cerebrovascular function and structure are largely attributed to modifiable risks factors that impact on cardiometabolic health, particularly physical inactivity (Ahtiluoto et al. 2010; Anstey et al. 2014; Bunch et al. 2010; Kalmijn et al. 2002; Kokmen et al. 1996; Kuller et al. 2005; Prins et al. 2002; Sabia et al. 2018; Scarmeas et al. 2009; Seliger et al. 2004; Solomon et al. 2009). Physical inactivity, in conjunction with reduced cardiometabolic health, promotes and exacerbates the development of chronic low-grade systemic inflammation and reduced endothelial function (Toda 2012; Woods et al. 2011; Bangen et al. 2014; Toth et al. 2017; AIHW 2012). Since physical inactivity promotes increased adiposity and low-grade systemic inflammation, which, in turn, can drive reactive oxygen species production thus reducing endothelial function, it may further exacerbate and reduce cerebrovascular function due to a reduction in capillary density and increased arterial stiffness (i.e. reduced blood flow and vasoreactivity) (Bangen et al. 2014; Toth et al. 2017; Toda 2012; Woods et al. 2011).
Finally, the only other variable that was strongly correlated with the primary outcomes was education. When education was removed as a covariate from the ANCOVA and all other variables mentioned used in this modelling (described above) were used as covariates (i.e. when we just removed education from the analysis), the results were still not significant between the groups. Currently, there is limited work, if any, that has determined the effects of education level on cerebrovascular function other than in Alzheimer’s disease, where it has been reported that higher regional CBF is associated with higher levels of education in these individuals (Chiu et al. 2004). However, more studies are required to determine the effect of education level on cerebrovascular function. Education duration has been correlated to increased cognitive capacity throughout the lifespan and as a potential attenuator of cognitive decline associated with ageing (Lövdén et al. 2020). Therefore, the impact of this on cognitive outcomes in this study cannot be excluded, even though an individual’s level of education is considered by the NIH Toolbox’s scoring algorithm (i.e. education is controlled for by the NIH Toolbox) (Heaton et al. 2014; Weintraub et al. 2014; Slotkin et al. 2012). Educational differences may also be an inherent complication with recruiting individuals for human trials, as the compliance and motivation to participate in studies appears to be higher in those who are more educated compared with those that are not (Mbuagbaw et al. 2017). This may pose a challenge to control for not only in this study but others as well, as others have highlighted education as an important that may affect clinical trials associated with neurodegenerative diseases, such as dementia (Huang et al. 2020). Interestingly, those with higher education levels also appear to progress more rapidly through the course of Alzheimer’s disease compared with those with lower educational levels (Kemppainen et al. 2008), meaning that the relationship of education and cognitive decline, particularly in relation to dementias, still requires more investigation. In any case, our results suggest that, while education is a significant element in maintaining overall brain health, having a favourable cardiometabolic profile is just as important in maintaining both cerebrovascular and cognitive function and that all of these elements are probably inter-related when it comes to improving or maintaining overall brain health throughout the lifespan.
Methodological limitations
Different techniques have been used to assess cerebrovascular function, such as direct methods like magnetic resonance imaging (MRI) and indirect methods such as TCD (see (Joris et al. 2018) for detailed comparison). CVR is a quantitative measure of the potential of the cerebral microcirculation to dilate in response to physical or chemical stimuli and is a surrogate measure of local endothelial function (Joris et al. 2018; Rossman et al. 2018). It is not a direct method in determining CBF differences in specific regions of the brain in response to a stimulus such as a cognitive task. We acknowledge that CVR is a crude measure that records changes essentially in one vessel only, which in this case is the MCA. We chose to use the MCA as changes in its blood flow velocity more directly reflect changes in CBF arising from the dilatation of the downstream microvasculature supplying brain regions that are critical for cognitive function (Braz et al. 2017; Serrador et al. 2000; Willie et al. 2011). Certain responses to different tests may not have been captured using TCD and by not excluding participants on the basis laterality. TCD and the use of the MCA only may not explain the magnitude differences in one test compared to another in specific brain regions (Joris et al. 2018). This may be seen as a limitation of the technique and by the fact we included participants who had either a bilateral or unilateral signal. Since we averaged the left- and right-side measures we may be reporting a reduced CVR in certain tasks. However, we captured the change in CVR without the exclusion of unilaterality, and, therefore, may not be a limitation, because this may have underestimated the associations between primary outcomes and the covariates. Additionally, it would not be expected that training status influences changes unilaterality, rather globally. More highly localised and direct processes could be used in future studies, if available, and these may give more definitive results. However, it is unlikely that this should be a limiting factor given that correlations between direct methods of CBF determination and TCD have been positively associated and validated (Miyazawa et al. 2018; Willie et al. 2011).
What was interesting in this study and has already been highlighted as a potential limitation above, is that the trained group had a higher level of education than the untrained. While others have reported this previously (Dishman et al. 1985; Lawrence 2017), there is also conjecture if this is limited to major metropolitan areas and extends to regionals areas. In Australia, particularly Queensland, metropolitan areas tend to be both more educated and physically active (QHealth 2020). It may be that educational level is a mediator of performing exercise, as education may be an important mediator of motivation to perform exercise (Dishman et al. 1985; Lawrence 2017). Alternatively, it may be that participating in exercise promotes or motivates individuals to further their education (Dishman et al. 1985) However, this differs regionally and rurally, where those in regional areas may be more educated than some areas but are less physically active than areas of lower education (QHealth 2020). In these areas, education level may not mediate exercise participation. This may account for why there were differences in education level in our participants, as we recruited a mix of people from metropolitan and regional areas. Determining sociodemographic differences were outside the scope of this study but may be an opportunity for future studies to perform these analyses within a single location and compare the differences between metropolitan, regional and rural centres.
A greater adiposity may contribute to a reduced cerebrovascular and cognitive function, independent of exercise training status (Bangen et al. 2014; Toda 2012; Toth et al. 2017; Woods et al. 2011). Obesity and sedentary behaviour rates are growing and may lead to the development of comorbidities, including cognitive decline and dementia (Taylor 2014; Brown et al. 2017; Beydoun et al. 2008). The untrained group in our study had a higher body mass, hip circumference, waist circumference, total body fat and hip-to-waist ratio than the trained group. We did not match the groups by body mass, BMI, waist circumference or body composition which is different from previous studies that reported no differences in BMI between the trained and untrained groups (Ainslie et al. 2008; Barnes et al. 2013; Braz et al. 2017; Miller et al. 2018; Zhu et al. 2013). We chose to not undertake this because our aim was to compare differences between older adults that had undertaken regular aerobic exercise training and those that were sedentary and untrained. As a result, the sedentary and untrained individuals had a higher a central adiposity and this was reflective of sedentary individuals in the regional areas of Australia where they were recruited. Given the high rates of physical inactivity and obesity globally, our aim was to replicate the real-world scenario and replicate this by comparing the two groups used in this study (OECD 2020). Additionally, there are variations in hypercapnia protocols, including breath-holding and manual manipulation, step-wised induction against non-stepwise induction and variation in baseline breathing (Ainslie et al. 2008; Barnes et al. 2012, 2013; Braz et al. 2017; Brown et al. 2010; Marley et al. 2020; Miller et al. 2018; Rogers et al. 1990, 1985; Zhu et al. 2013). We chose to use 5% carbon dioxide to induce hypercapnia, as this is a reliable challenge that we have undertaken previously (Evans et al. 2017a; Wong et al. 2016a, 2016b, 2016c). Finally, we acknowledge that our data may be confounded by other sources of variation such as diet and sex differences between participants. However, we attempted to minimise the latter by matching each group with an equal number of men and women.
Conclusion
In conclusion, older adults that had undertaken regular aerobic exercise training over the past 40 years had higher cerebrovascular and cognitive functions than age and sex matched, untrained and sedentary adults and their overall cognitive performance correlated moderately with their CVR to hypercapnia and strongly with their CVR to cognitive stimuli. However, following adjustment for covariates, CVR to hypercapnia, CVR to cognitive stimuli and total composite cognitive score did not differ significantly between trained and untrained groups. Our novel data demonstrate that there is a relationship between cerebrovascular and cognitive function in older adults and that there is an interaction between exercise training and cardiometabolic factors that may directly influence cerebrovascular and cognitive functions. Future studies are needed to examine the specific role of each these variables and how they are modulated by exercise to maintain cerebrovascular and cognitive function in older adults.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M (2010) Diabetes, Alzheimer disease, and vascular dementia. Neurology 75(13):1195
AIHW (2012) Dementia in Australia. AGE 70 edn. Australian Institute of Health and Welfare, Canberra
AIHW (2018) Physical activity across the life stages. Australian Institute of Health and Welfare, Canberra
Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJA, Atkinson G (2008) Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586(16):4005–4010. https://doi.org/10.1113/jphysiol.2008.158279
Anderson-Hanley C, Arciero PJ, Brickman AM, Nimon JP, Okuma N, Westen SC, Merz ME, Pence BD, Woods JA, Kramer AF, Zimmerman EA (2012) Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med 42(2):109–119. https://doi.org/10.1016/j.amepre.2011.10.016
Anstey KJ, Kingston A, Kiely KM, Luszcz MA, Mitchell P, Jagger C (2014) The influence of smoking, sedentary lifestyle and obesity on cognitive impairment-free life expectancy. Int J Epidemiol 43(6):1874–1883. https://doi.org/10.1093/ije/dyu170
ATS (2002) ATS statement: guidelines for the six-minute walk test. ATS committee on proficiency standards for clinical pulmonary function laboratories. Am J Respir Crit Care Med 166:111–117
Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN (2013) Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44(11):3235–3238. https://doi.org/10.1161/STROKEAHA.113.002589
Baker LD, Frank LL, Foster-Schubert K et al (2010) Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 67(1):71–79. https://doi.org/10.1001/archneurol.2009.307
Bangen KJ, Nation DA, Clark LR, Harmell AL, Wierenga CE, Dev SI, Delano-Wood L, Zlatar ZZ, Salmon DP, Liu TT, Bondi MW (2014) Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2014.00159
Barbour JA, Howe PRC, Buckley JD, Bryan J, Coates AM (2017) Cerebrovascular and cognitive benefits of high-oleic peanut consumption in healthy overweight middle-aged adults. Nutr Neurosci 20(10):555–562. https://doi.org/10.1080/1028415X.2016.1204744
Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ (2012) Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol 112(11):1884–1890. https://doi.org/10.1152/japplphysiol.01270.2011
Barnes JN, Taylor JL, Kluck BN, Johnson CP, Joyner MJ (2013) Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol 114(10):1383–1387. https://doi.org/10.1152/japplphysiol.01258.2012
BD (2019) Specimen Collection Resource Library [Internet]. Becton, Dickinson and Company. https://www.bd.com/en-us/offerings/capabilities/specimen-collection/specimen-collection-resource-library. Accessed July 2019
Beydoun MA, Beydoun HA, Wang Y (2008) Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev 9(3):204–218. https://doi.org/10.1111/j.1467-789X.2008.00473.x
Bliss ES, Wong RH, Howe PR, Mills DE (2021) Benefits of exercise training on cerebrovascular and cognitive function in ageing. J Cereb Blood Flow Metab 41(3):447–470. https://doi.org/10.1177/0271678x20957807
Bossers WJR, van der Woude LHV, Boersma F, Hortobágyi T, Scherder EJA, van Heuvelen MJG (2015) A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: a randomized, controlled trial. Am J Geriatr Psychiatry 23(11):1106–1116. https://doi.org/10.1016/j.jagp.2014.12.191
Braz ID, Flück D, Lip GYH, Lundby C, Fisher JP (2017) Impact of aerobic fitness on cerebral blood flow and cerebral vascular responsiveness to CO2 in young and older men. Scand J Med Sci Sports 27(6):634–642. https://doi.org/10.1111/sms.12674
Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ (2010) Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 31(12):2047–2057. https://doi.org/10.1016/j.neurobiolaging.2008.11.002
Brown L, Hansnata E, La HA (2017) Economic cost of dementia in Australia. Alzheimer’s Australia, Canberra
Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD (2010) Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm 7(4):433–437. https://doi.org/10.1016/j.hrthm.2009.12.004
Chiu N-T, Lee B-F, Hsiao S, Pai M-C (2004) Educational level influences regional cerebral blood flow in patients with Alzheimer’s disease. J Nucl Med 45(11):1860–1863
Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER (1993) A survey for assessing physical activity among older adults. Med Sci Sports Exerc 25(5):628–642 PMID: 8492692
Dishman RK, Sallis JF, Orenstein DR (1985) The determinants of physical activity and exercise. Public Health Rep 100(2):158–171
Duchemin S, Boily M, Sadekova N, Girouard H (2012) The complex contribution of NOS interneurons in the physiology of cerebrovascular regulation. Front Neural Circ. https://doi.org/10.3389/fncir.2012.00051
Edmonds HL Jr, Isley MR, Sloan TB, Alexandrov AV, Razumovsky AY (2011) American Society of Neurophysiologic Monitoring and American Society of Neuroimaging joint guidelines for Transcranial Doppler ultrasonic monitoring. J Neuroimaging 21(2):177–183. https://doi.org/10.1111/j.1552-6569.2010.00471.x
Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci 108(7):3017–3022. https://doi.org/10.1073/pnas.1015950108
Evans H, Howe P, Wong R (2017a) Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 9(1):27
Evans HM, Howe PR, Wong RH (2017) Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients. https://doi.org/10.3390/nu9010027
Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G (2014) Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage 85:592–607
Friedenreich CM, Courneya KS, Bryant HE (1998) The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc 30(2):266–274
Hajjar I, Marmerelis V, Shin DC, Chui H (2014) Assessment of cerebrovascular reactivity during resting state breathing and its correlation with cognitive function in hypertension. Cerebrovasc Dis 38(1):10–16
Harada CN, Natelson Love MC, Triebel KL (2013) Normal cognitive aging. Clin Geriatr Med 29(4):737–752. https://doi.org/10.1016/j.cger.2013.07.002
Harris M (2013) The metabolic syndrome. Aust Fam Physician 42:524–527
Harris S, Reyhan T, Ramli Y, Prihartono J, Kurniawan M (2018) Middle cerebral artery pulsatility index as predictor of cognitive impairment in hypertensive patients. Front Neurol. https://doi.org/10.3389/fneur.2018.00538
Harvey PD (2019) Domains of cognition and their assessment. Dialogues Clin Neurosci 21(3):227–237. https://doi.org/10.31887/DCNS.2019.21.3/pharvey
Healthineers (2019) ADVIA Chemistry XPT System. Siemens Healthcare. https://www.siemens-healthineers.com/clinical-chemistry/systems/advia-chemistry-xpt-system. Accessed July 2019
Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, Beaumont J, Casaletto KB, Conway K, Slotkin J, Gershon R (2014) Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc 20(6):588–598. https://doi.org/10.1017/s1355617714000241
Heuchert JP, McNair DM (2012) Profile of mood states 2nd edition™: POMS 2. Multi-Health Systems Inc., North Tonawanda
Hillman TE, Nunes QM, Hornby ST, Stanga Z, Neal KR, Rowlands BJ, Allison SP, Lobo DN (2005) A practical posture for hand grip dynamometry in the clinical setting. Clin Nutr 24(2):224–228. https://doi.org/10.1016/j.clnu.2004.09.013
Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, Brændgaard H, Gottrup H, Lolk A, Wermuth L, Jacobsen S, Laugesen LP, Gergelyffy RG, Høgh P, Bjerregaard E, Andersen BB, Siersma V, Johannsen P, Cotman CW, Waldemar G, Hasselbalch SG (2016) Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimers Dis 50(2):443–453. https://doi.org/10.3233/jad-150817
Huang LK, Chao SP, Hu CJ (2020) Clinical trials of new drugs for Alzheimer disease. J Biomed Sci 27(1):18. https://doi.org/10.1186/s12929-019-0609-7
Jialal I, Devaraj S (2001) Inflammation and atherosclerosis: the value of the high-sensitivity C-reactive protein assay as a risk marker. Am J Clin Pathol 116(Suppl):S108-115. https://doi.org/10.1309/j63v-5lth-wyfc-vdr5
Johansson H, Hagströmer M, Grooten WJA, Franzén E (2020) Exercise-induced neuroplasticity in Parkinson’s disease: a metasynthesis of the literature. Neural Plast 2020:8961493. https://doi.org/10.1155/2020/8961493
Joris PJ, Mensink RP, Adam TC, Liu TT (2018) Cerebral blood flow measurements in adults: a review on the effects of dietary factors and exercise. Nutrients 10(5):530. https://doi.org/10.3390/nu10050530
Kalmijn S, van Boxtel MPJ, Verschuren MWM, Jolles J, Launer LJ (2002) Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol 156(10):936–944. https://doi.org/10.1093/aje/kwf135
Keage HA, Kurylowicz L, Lavrencic LM, Churches OF, Flitton A, Hofmann J, Kohler M, Badcock NA (2015) Cerebrovascular function associated with fluid, not crystallized, abilities in older adults: a transcranial Doppler study. Psychol Aging 30(3):613
Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, Johnson NA (2015) Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 63(1):174–182. https://doi.org/10.1016/j.jhep.2015.02.022
Kemppainen NM, Aalto S, Karrasch M, Någren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO (2008) Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol 63(1):112–118. https://doi.org/10.1002/ana.21212
Kennedy KM, Raz N (2015) Normal aging of the brain. In: Toga AW (Eds) Brain mapping. Academic Press, Waltham, pp 603–617. https://doi.org/10.1016/B978-0-12-397025-1.00068-3
Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL (1972) Indices of relative weight and obesity. J Chronic Dis 25(6–7):329–343. https://doi.org/10.1016/0021-9681(72)90027-6
Kokmen E, Whisnant JP, Fallon WM, Chu CP, Beard CM (1996) Dementia after ischemic stroke. Neurology 46(1):154
Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, Kawas C, Breitner JCS, Fitzpatrick A, Dulberg C (2005) Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64(9):1548
Lam LC, Chan WC, Leung T, Fung AW, Leung EM (2015) Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: a cluster randomized controlled trial. PLoS One 10(3):e0118173. https://doi.org/10.1371/journal.pone.0118173
Lautenschlager NT, Cox KL, Flicker L et al (2008) Effect of physical activity on cognitive function in older adults at risk for alzheimer disease: a randomized trial. J Am Med Assoc 300(9):1027–1037. https://doi.org/10.1001/jama.300.9.1027
Lawrence EM (2017) Why do college graduates behave more healthfully than those who are less educated? J Health Soc Behav 58(3):291–306. https://doi.org/10.1177/0022146517715671
Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y (2015) Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 4(7):e002014. https://doi.org/10.1161/JAHA.115.002014
Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM (2020) Education and cognitive functioning across the life span. Psychological Science in the Public Interest 21(1):6–41. https://doi.org/10.1177/1529100620920576
Marley CJ, Brugniaux JV, Davis D, Calverley TA, Owens TS, Stacey BS, Tsukamoto H, Ogoh S, Ainslie PN, Bailey DM (2020) Long-term exercise confers equivalent neuroprotection in females despite lower cardiorespiratory fitness. Neuroscience 427:58–63. https://doi.org/10.1016/j.neuroscience.2019.12.008
Mbuagbaw L, Aves T, Shea B, Jull J, Welch V, Taljaard M, Yoganathan M, Greer-Smith R, Wells G, Tugwell P (2017) Considerations and guidance in designing equity-relevant clinical trials. Int J Equity Health 16(1):93. https://doi.org/10.1186/s12939-017-0591-1
Miller KB, Howery AJ, Harvey RE, Eldridge MW, Barnes JN (2018) Cerebrovascular reactivity and central arterial stiffness in habitually exercising healthy adults. Front Physiol. https://doi.org/10.3389/fphys.2018.01096
Miyazawa T, Shibata S, Nagai K, Hirasawa A, Kobayashi Y, Koshiba H, Kozaki K (2018) Relationship between cerebral blood flow estimated by transcranial Doppler ultrasound and single photon emission computed tomography in elderly with dementia. J Appl Physiol 125(5):1576–1584. https://doi.org/10.1152/japplphysiol.00118.2018
Mohammadi H, Gagnon C, Vincent T, Kassab A, Fraser S, Nigam A, Lesage F, Bherer L (2021) Longitudinal impact of physical activity on brain pulsatility index and cognition in older adults with cardiovascular risk factors: a NIRS study. Brain Sci 11(6):730
Nealon RS, Howe PR, Jansen L, Garg M, Wong RH (2017) Impaired cerebrovascular responsiveness and cognitive performance in adults with type 2 diabetes. J Diabetes Complic 31(2):462–467. https://doi.org/10.1016/j.jdiacomp.2016.06.025
Ngandu T, Lehtisalo J, Korkki S, Solomon A, Coley N, Antikainen R, Bäckman L, Hänninen T, Lindström J, Laatikainen T, Paajanen T, Havulinna S, Peltonen M, Neely AS, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M (2022) The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimers Dement 18(7):1325–1334. https://doi.org/10.1002/alz.12492
OECD (2020) World Health Organization: overweight and obesity. https://doi.org/10.1787/a47d0cd2-en
Pannucci TE, Thompson FE, Bailey RL, Dodd KW, Potischman N, Kirkpatrick SI, Alexander GL, Coleman LA, Kushi LH, Groesbeck M, Sundaram M, Clancy H, George SM, Kahle L, Subar AF (2018) Comparing reported dietary supplement intakes between two 24-Hour recall methods: the automated self-administered 24-hour dietary assessment tool and the interview-administered automated multiple pass method. J Acad Nutr Diet 118(6):1080–1086. https://doi.org/10.1016/j.jand.2018.02.013
Paterson DH, Jones GR, Rice CL (2007) Advancing physical activity measurement and guidelines in Canada: a scientific review and evidence-based foundation for the future of Canadian physical activity guidelines. Appl Physiol Nutr Metab 32:75–121. https://doi.org/10.1139/h07-923
Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Jolles J, Clarke R, Breteler MMB (2002) Homocysteine and cognitive function in the elderly. Neurology 59(9):1375
Prisant LM, Pasi M, Jupin D, Prisant ME (2002) Assessment of repeatability and correlates of arterial compliance. Blood Press Monit 7(4):231–235
QHealth (2020) The health of Queenslanders 2020. Report of the Chief Health Officer Queensland. Brisbane
QML (2019) QML Pathology Test Reference Manual [Internet]. QML Pathology. http://www.qml.com.au/IamaDoctor/TestingGuide/ReferenceManual.aspx. Accessed July 2019
RCPA (2021) RCPA Manual: Pathology Tests. RCPA. https://www.rcpa.edu.au/Manuals/RCPA-Manual/Pathology-Tests. Accessed 2021
Richiardi J, Monsch AU, Haas T, Barkhof F, Van de Ville D, Radü EW, Kressig RW, Haller S (2015) Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 36(1):33–41
Rogers RL, Meyer JS, Mortel KF, Mahurin RK, Thornby J (1985) Age-related reductions in cerebral vasomotor reactivity and the law of initial value: a 4-year prospective longitudinal study. J Cereb Blood Flow Metab 5(1):79–85. https://doi.org/10.1038/jcbfm.1985.11
Rogers RL, Meyer JS, Mortel KF (1990) After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc 38(2):123–128. https://doi.org/10.1111/j.1532-5415.1990.tb03472.x
Rossman MJ, LaRocca TJ, Martens CR, Seals DR (2018) Healthy lifestyle-based approaches for successful vascular aging. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00521.2018
Ruscica M, Ferri N, Banach M, Sirtori CR, Corsini A (2022) Side effects of statins-from pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc Res. https://doi.org/10.1093/cvr/cvac020
Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, Kivimäki M, Singh-Manoux A (2018) Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 362:k2927. https://doi.org/10.1136/bmj.k2927. PMID: 30068508; PMCID: PMC6066998
Salthouse T (2012) Consequences of age-related cognitive declines. Annu Rev Psychol 63(1):201–226. https://doi.org/10.1146/annurev-psych-120710-100328
Scarmeas N, Luchsinger JA, Schupf N et al (2009) Physical activity, diet, and risk of Alzheimer disease. J Am Med Assoc 302(6):627–637. https://doi.org/10.1001/jama.2009.1144
Schober P, Boer C, Schwarte LA (2018) Correlation coefficients: appropriate use and interpretation. Anesth Analg 126(5):1763–1768. https://doi.org/10.1213/ane.0000000000002864
Seliger SL, Siscovick DS, Stehman-Breen CO, Gillen DL, Fitzpatrick A, Bleyer A, Kuller LH (2004) Moderate renal impairment and risk of dementia among older adults: the cardiovascular health cognition study. J Am Soc Nephrol 15(7):1904
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31(7):1672–1678
Slotkin J, Nowinski C, Hays R, Beaumont J, Griffith J, Magasi S, Salsman J, Gershon R (2012) NIH Toolbox scoring and interpretation guide. National Institutes of Health, Washington (DC), pp 6–7
Sobol NA, Hoffmann K, Vogel A, Lolk A, Gottrup H, Høgh P, Hasselbalch SG, Beyer N (2016) Associations between physical function, dual-task performance and cognition in patients with mild Alzheimer’s disease. Aging Ment Health 20(11):1139–1146. https://doi.org/10.1080/13607863.2015.1063108
Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA (2009) Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 28(1):75–80. https://doi.org/10.1159/000231980
Strauss E, Sherman E, Spreen O (2006) A compendium of neuropsychological tests, 3rd edn. Oxford University Press, New York
Taylor D (2014) Physical activity is medicine for older adults. Postgrad Med J 90(1059):26–32. https://doi.org/10.1136/postgradmedj-2012-131366
Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H (2013) Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 38(5):1177–1183. https://doi.org/10.1002/jmri.24090
Toda N (2012) Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther 133(2):159–176. https://doi.org/10.1016/j.pharmthera.2011.10.004
Toth P, Tarantini S, Csiszar A, Ungvari Z (2017) Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol-Heart Circ Physiol 312(1):H1–H20. https://doi.org/10.1152/ajpheart.00581.2016
Vaynman S, Ying Z, Gomez-Pinilla F (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20(10):2580–2590. https://doi.org/10.1111/j.1460-9568.2004.03720.x
Vicente-Campos D, Mora J, Castro-Piñero J, González-Montesinos JL, Conde-Caveda J, Chicharro JL (2012) Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness 52(5):537–544
Vreugdenhil A, Cannell J, Davies A, Razay G (2012) A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci 26(1):12–19. https://doi.org/10.1111/j.1471-6712.2011.00895.x
Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Slotkin J, Carlozzi NE, Bauer PJ, Wallner-Allen K, Fox N, Havlik R, Beaumont JL, Mungas D, Manly JJ, Moy C, Conway K, Edwards E, Nowinski CJ, Gershon R (2014) The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc 20(6):567–578. https://doi.org/10.1017/s1355617714000320
Welborn TA, Dhaliwal SS, Bennett SA (2003) Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust 179(11/12):580–585
Wijnhoud AD, Koudstaal PJ, Dippel DW (2011) The prognostic value of pulsatility index, flow velocity, and their ratio, measured with TCD ultrasound, in patients with a recent TIA or ischemic stroke. Acta Neurol Scand 124(4):238–244. https://doi.org/10.1111/j.1600-0404.2010.01462.x
Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN (2011) Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196(2):221–237. https://doi.org/10.1016/j.jneumeth.2011.01.011
Wong R, Raederstorff D, Howe P (2016a) Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 8(7):425
Wong RH, Evans HM, Howe PR (2016b) Poor cerebrovascular function is an early marker of cognitive decline in healthy postmenopausal women. Alzheimer’s Dementia Transl Res Clin Interv 2(3):162–168
Wong RHX, Nealon RS, Scholey A, Howe PRC (2016c) Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 26(5):393–399. https://doi.org/10.1016/j.numecd.2016.03.003
Wong R, Al-Omary M, Baker D, Spratt N, Boyle A, Baker N, Howe P, Collins N (2019) Cognitive dysfunction is associated with abnormal responses in cerebral blood flow in patients with single ventricular physiology: Novel insights from transcranial Doppler ultrasound. Congenit Heart Disease 14(4):638–644. https://doi.org/10.1111/chd.12763
Woods JA, Wilund KR, Martin SA, Kistler BM (2011) Exercise, inflammation and aging. Aging Dis 3(1):130–140
You T, Arsenis NC, Disanzo BL, Lamonte MJ (2013) Effects of exercise training on chronic inflammation in obesity : Current evidence and potential mechanisms. Sports Med 43(4):243–256. https://doi.org/10.1007/s40279-013-0023-3
Zhu Y-S, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R (2013) Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and masters athletes. J Cereb Blood Flow Metab 33(8):1190–1196. https://doi.org/10.1038/jcbfm.2013.66
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723. https://doi.org/10.1038/nrn3114
Acknowledgements
The authors would like to acknowledge the University of Southern Queensland for assistance in funding the production of this manuscript. The authors would also like to thank QML Pathology for their timely reporting of some of the biochemical analyses performed at their laboratory. We would also like to thank all study participants for their time and effort to ensure this project was completed.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was supported in part by a University of Southern Queensland Strategic Funding Grant. ESB was supported by an Australian Postgraduate Award.
Author information
Authors and Affiliations
Contributions
ESB and DEM conceptualised and designed the study protocol. RHXW, PRCH, ESB and DEM designed the experiments. ESB and SMB collected the data. ESB and SMB analysed the data. ESB and DEM performed statistical analysis, with all authors contributing to data interpretation. ESB, SMB, RHXW, PRCH and DEM contributed to revisions of intellectual content. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Lori Ann Vallis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bliss, E.S., Biki, S.M., Wong, R.H.X. et al. The benefits of regular aerobic exercise training on cerebrovascular function and cognition in older adults. Eur J Appl Physiol 123, 1323–1342 (2023). https://doi.org/10.1007/s00421-023-05154-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05154-y