Abstract
Purpose
To determine the effect of prolonged exercise on moderate-to-heavy intensity transition power output and heart rate.
Methods
Fourteen endurance-trained cyclists and triathletes took part in the present investigation (13 males, 1 female, V·O2peak 59.9 ± 6.8 mL.kg−1.min−1). Following a characterisation trial, participants undertook a five-stage incremental step test to determine the power output and heart rate at the moderate-to-heavy intensity transition before and after two hours of cycling at 90% of the estimated power output at first ventilatory threshold (VT1).
Results
Power output at the moderate-to-heavy intensity transition significantly decreased following acute prolonged exercise when determined using expired gases (VT1, 217 ± 42 W vs. 196 ± 42 W, P < 0.0001) and blood lactate concentrations (LoglogLT, 212 ± 47 W vs. 190 ± 47 W, P = 0.004). This was attributable to loss of efficiency (VT1, -8 ± 10 W; LoglogLT, − 7 ± 9 W) and rates of metabolic energy expenditure at the transition (VT1, − 14 ± 11 W; LoglogLT, − 15 ± 22 W). The heart rate associated with the moderate-to-heavy intensity transition increased following acute prolonged exercise (VT1, 142 ± 9 beats.min−1 vs. 151 ± 12 beats.min−1, P < 0.001; LoglogLT, 140 ± 13 beats.min−1 vs. 150 ± 15 beats.min−1, P = 0.006).
Conclusion
These results demonstrate the external work output at the moderate-to-heavy intensity transition decreases during prolonged exercise due to decreased efficiency and rates of metabolic energy expenditure, but the associated heart rate increases. Therefore, individual assessments of athlete ‘durability’ are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endurance athletes commonly perform physiological assessments for estimating the external work outputs that demarcate the boundaries between exercise intensity domains. These intensity domain transitions are used to assess performance capability, regulate training and competition intensities, monitor training load, and quantify adaptations to training (Maunder et al. 2021). The moderate and heavy exercise intensity domains are defined by physiological responses, including distinct blood lactate and whole-body oxygen uptake (V·O2) kinetics profiles (Burnley and Jones 2018; Jones et al. 2019).
An under-studied effect of prolonged exercise is the likely reduction in external work rates observed at the intensity transitions over time; the resilience to which we termed ‘durability’ (Maunder et al. 2021). A series of recent studies reported a reduction in the so-called critical power following prolonged heavy-intensity cycling, and indicated that the magnitude of the reduction was sensitive to carbohydrate availability (Clark et al. 2018, 2019a, b). Less is understood regarding the effects of acute prolonged exercise on the moderate-to-heavy intensity transition, which may plausibly also decline with prolonged exercise in a manner related to carbohydrate availability. Specifically, depletion of the intramyofibrillar glycogen store during prolonged exercise appears to impair muscle contractile function (Ørtenblad et al. 2013). Therefore, following prolonged exercise, intramyofibrillar glycogen depletion-induced impairment of specific muscle fibres may increase the mechanical and metabolic burden of a given power output on a smaller pool of fully functional fibres. Speculatively, this could increase the fibre-specific work rate at a given power output, reducing the power output achieved at the moderate-to-heavy intensity transition following prolonged exercise.
A reduction in gross cycling efficiency with prolonged moderate-intensity exercise has been observed (Passfield and Doust 2000; Hopker et al. 2017). This would theoretically contribute to reduced power output at intensity transitions following prolonged exercise, even if the rate of metabolic energy expenditure associated with the transitions is maintained over time. The contributions made by changes in gross cycling efficiency and rates of metabolic energy expenditure to prolonged exercise-induced changes in the power output at the moderate-to-heavy transition could be quantified by measurement of the energy expenditure vs. power output relationship, and identification of the rate of energy expenditure associated with the moderate-to-heavy intensity transition, before and after prolonged exercise.
Given the possibility that power outputs at intensity domain transitions may decrease during prolonged exercise, the use of data generated in well-rested physiological profiling assessments to regulate training intensity could result in inadvertent drift into higher intensity domains over time (Maunder et al. 2021). A common strategy employed by endurance athletes to combat this is the use of heart rates observed at intensity transitions to regulate training intensity. As progressive increases in heart rate at given power outputs can occur during prolonged exercise (Coyle and Gonzalez-Alonso 2001), an athlete using heart rate for training intensity regulation may progressively reduce their power output to remain at the target heart rate. Surprisingly, the degree to which cardiovascular drift during prolonged exercise reflects shifts in intensity domain transitions has not been established. Understanding the relationship between cardiovascular drift and acute exercise-induced changes in the power outputs at intensity domain transitions has implications for how heart rate monitoring might be used for training intensity regulation during prolonged exercise.
Therefore, the aims of the present investigation were to: (i) quantify the effects of prolonged cycling on the power output at the moderate-to-heavy intensity transition, (ii) determine the contributions made by changes in gross cycling efficiency and rates of metabolic energy expenditure at the moderate-to-heavy transition to prolonged cycling-induced changes in moderate-to-heavy intensity transition power output, and (iii) quantify the effects of prolonged cycling on the heart rate associated with the moderate-to-heavy intensity transition. We hypothesised that moderate-to-heavy transition power output would decrease following prolonged cycling due to decreased gross cycling efficiency and rates of metabolic energy expenditure at the transition, but that the heart rate associated with the transition would remain consistent over time.
Methods
Ethical approval
This study was performed in accordance with the standards of the Declaration of Helsinki, 2013. The Auckland University of Technology Ethics Committee approved all procedures (21/253), and all participants provided written informed consent prior to participation. This study was not registered in a database. Data associated with this study are available from the corresponding author upon reasonable request.
Participants
Fourteen endurance-trained cyclists and triathletes took part in the present investigation (13 males, 1 female, age 34 ± 10, height 178.1 ± 5.6 cm, mass 71.2 ± 6 kg, V·O2peak 59.9 ± 6.8 mL.kg−1.min−1, training volume 9 ± 3 h.week−1). A priori sample size estimation indicated that a total sample size of 15 was required to detect a large magnitude (ES = 0.7) reduction in power output at the moderate-to-heavy intensity transition with 80% statistical power using the G*Power software package. A large magnitude effect size was used for this calculation based on previous studies showing the effect of prolonged exercise on the heavy-to-severe intensity transition (Clark et al. 2018, 2019a, b). A one-tailed test was used as it seemed implausible that the moderate-to-heavy intensity power output would increase following acute prolonged exercise. Data collection was interrupted by a COVID-19 lockdown and one participant dropped out. All participants were free of recent (< 3 months) musculoskeletal injury and chronic disease and habitually training > 5 h.week−1 in cycling-based endurance sports. This study was performed in accordance with the standards of the Declaration of Helsinki, 2013, and the Auckland University of Technology Ethics Committee approved all procedures (21/253).
Study design
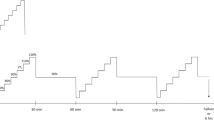
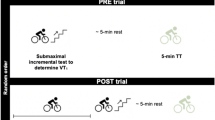
Participants visited the laboratory on two occasions, ~ 7 d apart, for: (i) a characterisation trial involving a maximal, incremental cycling test after an overnight fast and (ii) a prolonged trial, which involved a prolonged cycling assessment with estimation of the first ventilatory threshold (VT1) before and after two hours of moderate-intensity cycling, after an overnight fast (Fig. 1). The order of visits was not randomised as the incremental test data were used to define the parameters of the prolonged trial.
Characterisation trial
Participants initially reported to the laboratory for an incremental cycling test. Participants arrived after a 10-h overnight fast having refrained from vigorous exercise for 24 h and having ingested ~ 1 L of plain water ~ 2 h beforehand. Height and body mass was first measured. Cycling subsequently commenced on an electromagnetically braked ergometer at 95 W, and the power output initially increased by 35 W every 3 min (Excalibur Sport, Lode BV, Groningen, NET). Expired gases were collected continuously using indirect calorimetry (TrueOne 2400, ParvoMedics, UT, USA). Heart rate was measured continuously using a chest-strap heart rate monitor (Tickr, Wahoo Fitness, Atlanta, USA).
Once the respiratory exchange ratio exceeded 1.0 and clear signs of increased V̇E.V·̇O2−1 emerged, power output was increased by 35 W every minute until task failure. The V·̇O2peak was identified as the highest 15-s averageV·̇O2, and VT1 was identified as the V·̇O2 at which a systematic rise in V̇E.V·̇O2−1 occurred. This V·̇O2 was converted to a power output by linear fit of the power output vs.V·̇O2 relationship, using the last minute of V·̇O2 data from each 3-min stage.
The last minute of expired gas data in each 3-min stage was used to determine whole-body fat oxidation rates through standard calculations (Jeukendrup and Wallis 2005) (Eq. 1). The highest observed rate of whole-body fat oxidation was accepted as the peak fat oxidation rate (PFO), as per our recent work (Maunder et al. 2022).
where V·̇O2 and V̇CO2 are in L.min−1.
Prolonged trial
Participants arrived for the prolonged trial after a 10-h overnight fast, having refrained from vigorous exercise for 24 h, and having ingested ~ 1 L of plain water ~ 2 h beforehand. Following measurement of body mass, the experimental trial commenced on the same electromagnetically braked ergometer as the initial assessment with a 5-min warm-up at 100 W, followed by a five-stage incremental assessment to determine the power output and heart rate at the moderate-to-heavy intensity transition. The incremental test began with 4-min at 50 W below the previously estimated VT1 power output, and power output increased by 25 W per increment.
Expired gases and heart rate were measured continuously (TrueOne 2400, ParvoMedics, UT, USA; Tickr, Wahoo Fitness, Atlanta, USA), and a fingerprick capillary blood sample was obtained in the last 30-s of each increment for measurement of blood lactate concentration using an automated analyser (Lactate Pro 2, Arkray). These data were used to quantify the power output and heart rate at the moderate-to-heavy intensity transition prior to prolonged exercise (PRE, see Data analysis section below). Participants then cycled for 5 min at 100 W, and then at 90% of the previously estimated power output at VT1 for 2 h. Heart rate was recorded throughout, and participants consumed plain water ad libitum. Expired gases were collected for 4 min, every 15 min.
Following the two-hour constant work-rate phase, participants again cycled for 5 min at 100 W before repeating the five-step incremental exercise assessment. These data were used to quantify the power output and heart rate at the moderate-heavy intensity transition following prolonged exercise (POST). Sweat loss was also assessed by measurement of pre- and post-exercise body mass, and water consumption. Total water consumption was recorded by measuring the mass of the bottle before and after use and refilling, and was then added to changes in body mass to calculate total sweat loss. If participants used the toilet during the trial, body mass was recorded before and after and accounted for in sweat loss calculations.
Estimation of the moderate-to-heavy transition
The PRE and POST moderate-to-heavy intensity transitions were estimated using expired gas and blood lactate data. Specifically, using expired gas data, the moderate-to-heavy intensity transitions in the PRE and POST assessments of the experimental trial were estimated using the VT1 method in accordance with the procedures described above for the initial assessment. The V·̇O2 at VT1 was converted to a power output by linear fit of the power output vs. V·̇O2 relationship, using the last minute of V·̇O2 data from each of the five 4-min stages. Power output was then matched with a heart rate value by linear fit of the power output vs. heart rate relationship, using the average heart rate during the last minute of each 4-min stage. The VT1 power output was then converted to a rate of whole-body energy expenditure by linear fit of the whole-body energy expenditure vs. power output relationship. The whole-body rate of energy expenditure was calculated for each power output in the incremental assessment using the average V·̇O2 and V̇CO2 in the last minute of each of the five 4-min stages with a stoichiometric equation (Jeukendrup and Wallis 2005) (Eq. 2)
Where V·̇O2 and V·̇O2 are in L.min−1.
Using blood lactate data, the PRE and POST moderate-to-heavy intensity transitions were estimated using the LoglogLT method. The LoglogLT method models a blood lactate concentration vs. power output curve using two segments, and the intersection point of the two lines with the lowest residuals sum of squares is taken as the moderate-to-heavy intensity transition (Jamnick et al. 2018). The LoglogLT power output in the PRE and POST assessments were converted to heart rate, V·̇O2, and whole-body rate of energy expenditure values by linear fit of the relationships between these values and power output, as per above. As LoglogLT data produced essentially the same results as VT1, and the same inferences, only VT1 data are reported. The LoglogLT data can be found in Supplementary Figs. 1–2.
To quantify the proportion of prolonged exercise-induced changes in moderate-to-heavy intensity transition power output associated with changes in gross cycling efficiency and changes in rates of metabolic energy expenditure achieved at the moderate-to-heavy transition, rates of energy expenditure observed at VT1 and LoglogLT in the POST assessment were converted to a power output value using linear regression of the power output vs. energy expenditure relationship for each participant in the PRE assessment (denoted as POSTEEPREEff). The POSTEEPREEff therefore indicates the power output that the rate of metabolic energy expenditure observed at the moderate-to-heavy transition in the POST assessment would have achieved with the level of gross cycling efficiency in the PRE assessment. Accordingly, the proportion of prolonged exercise-induced changes in VT1 and LoglogLT power output associated with changes in energetic efficiency and rates of metabolic energy expenditure achieved at the transition was calculated using the below equation (Eq. 3).
Equation 3 where PRE = power output at the moderate-to-heavy transition pre-prolonged exercise, POST = power output at the moderate-to-heavy transition post-prolonged exercise, and POSTEEPREEff = power output that would be produced in the PRE assessment using the rate of metabolic energy expenditure observed at the moderate-to-heavy transition in the POST assessment.
Statistical analysis
Data are presented as mean ± standard deviation (SD), unless otherwise stated. Normality of data distributions were assessed using the Shapiro–Wilk test. The effect of prolonged exercise on moderate-to-heavy intensity transition power output, heart rate, V·̇O2, and rate of energy expenditure was assessed using paired t-tests (or the non-parametric equivalent Wilcoxon test). Relationships between PRE to POST changes in moderate-to-heavy transition power output and PFO, V·̇O2peak, the moderate-to-heavy intensity transition in the PRE assessment, sweat loss, and dehydration were assessed using Pearson’s (r) or Spearman’s rank-order (rs) correlation coefficients, depending on normality, and expressed with 95% confidence intervals. Changes in heart rate, whole-body energy expenditure, V·̇O2, and respiratory exchange ratio (RER) over-time during the two-hour constant work-rate phase were analysed using repeated measures one-way analyses of variance. Whole-body fat oxidation rates during the first three stages of the PRE and POST incremental tests were compared using a mixed model analysis of variance due to missing data-points. Variance was located post-hoc using Holm-Bonferroni stepwise correction. Analyses were performed in GraphPad Prism Version 9.3.1. Significance was inferred when P ≤ 0.05.
Results
Constant work-rate phase
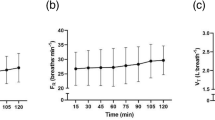
The estimated power output at VT1 in the initial assessment was 216 ± 45 W. Consequently, the two-hour constant work-rate phase in the experimental trial was completed at 194 ± 41 W. From 15 to 120 min of the two-hour constant work-rate phase, heart rate significantly increased (P < 0.0001, 8.2 ± 2.7%, Fig. 2a). Rates of whole-body energy expenditure (P = 0.07, 3.4 ± 4.3%, Fig. 2b) and V·̇O2 (P = 0.08, 3.4 ± 4.9%, Fig. 2c) did not increase during the two-hour constant work-rate phase, although both effects approached significance. The RER did not change during the two-hour constant work-rate phase (P = 0.61, Fig. 2d).
Moderate-to-heavy intensity transition
The power output at VT1 significantly decreased from PRE to POST (217 ± 42 W vs. 196 ± 42 W, ∆ − 21 ± 12 W, ∆ − 10.0 ± 5.8%, P < 0.0001, Fig. 3). The magnitude of PRE to POST change in VT1 power output was not significantly associated with PFO, V·̇O2peak, prolonged exercise-induced sweat loss, or prolonged exercise-induced dehydration (Table 1). However, the magnitude of the reduction in VT1 power output from PRE to POST was be related to the VT1 expressed as %V·̇O2peak in the PRE assessment.
The V·̇O2 at VT1 significantly decreased from PRE to POST (2.89 ± 0.55 vs. 2.69 ± 0.51 L.min−1, 68 ± 7 vs. 63 ± 8% of V·̇O2peak, P < 0.0001, Fig. 4a). The rate of energy expenditure at VT1 significantly decreased from PRE to POST (14.4 ± 2.7 vs. 13.5 ± 2.7 kcal.min−1, P = 0.0002, Fig. 4b). The heart rate at VT1 significantly increased from PRE to POST (142 ± 9 vs. 151 ± 12 beats.min−1, P = 0.001, Fig. 4c). The exercise-induced decrease in power output at VT1 was attributable to decreased energetic efficiency (-8 ± 10 W) and rates of metabolic energy expenditure at the transition (− 14 ± 11 W, Fig. 4d). The relative contribution made by decreased efficiency and rates of metabolic energy expenditure to the decrease in power output at VT1 was not significantly different (P = 0.18).
The a rate of oxygen consumption (V·̇O2), b energy expenditure (EE), and c heart rate (HR) at the first ventilatory threshold (VT1) before (PRE) and after (POST) prolonged exercise. The contributions to prolonged exercise-induced changes in VT1 power output made by loss of efficiency and metabolic energy expenditure at the transition is shown in d. Bars indicate mean values and lines indicate individual responses. *** denotes P ≤ 0.001, **** denotes P ≤ 0.0001
There was an effect of prolonged exercise (P = 0.04), and prolonged exercise by intensity interaction (P = 0.002), on whole-body fat oxidation rates during the PRE and POST assessments. Specifically, whole-body fat oxidation rates were greater in the POST vs. PRE assessment during the first and second steps (Fig. 5). The loss of power output at the moderate-to-heavy intensity transition associated with decreased energetic efficiency was significantly associated with changes in whole-body fat oxidation rates from PRE to POST, but these relationships were not present for the loss of power output associated with rates of metabolic energy expenditure at the transition (Table 2, Supplementary Fig. 3).
The whole-body fat oxidation rate (g.min−1) in steps 1, 2, and 3 of the incremental exercise tests before (PRE) and after (POST) the two-hour constant work-rate phase in the prolonged trial. Circles indicate means and error bars indicate standard deviations. Data is plotted for the first three steps as in some instances the respiratory exchange ratio approached or exceeded 1.0 in the fourth and fifth steps if the POST assessment. * denotes P ≤ 0.05 in PRE vs. POST
Discussion
The aim of this study was to determine the effects of prolonged moderate-intensity cycling on the moderate-to-heavy intensity transition power output and heart rate. Our primary observations were that: (i) the power output at the moderate-to-heavy intensity transition significantly decreased following prolonged cycling, (ii) this loss of power output was attributable to reduced gross cycling energetic efficiency and reduced rates of metabolic energy expenditure at the transition, and (iii) the heart rate associated with the moderate-to-heavy intensity transition increased following prolonged cycling. These data have implications for athlete profiling, training load monitoring, and training programming, and indicate that the ‘durability’ of the moderate-to-heavy intensity transition warrants attention at the individual level.
The observed reduction in moderate-to-heavy intensity transition power output following prolonged exercise was partially accounted for by reduced energetic efficiency, or the translation of metabolic energy expenditure to mechanical power output (Fig. 4d). Loss of efficiency was also demonstrated during the two-hour constant work-rate phase, as whole-body rates of energy expenditure (P = 0.07, Fig. 2b) and V·̇O2 (P = 0.08, Fig. 2b) at the fixed power output increased over-time, albeit not significantly. These observations are in line with previous work demonstrating decreased gross cycling efficiency following prolonged exercise (Passfield and Doust 2000; Hopker et al. 2017). Mechanistically, the rising energy and V·̇O2 cost of given power outputs following prolonged exercise may reflect progressive loss of skeletal muscle contractile efficiency due to increasing recruitment of less efficient type II muscle fibres (Jones et al. 2011). The increased V·̇O2 cost of producing a fixed power output would have been exacerbated beyond the loss of energetic efficiency by the increased fat oxidation (Fig. 5), as fat oxidation requires more V·̇O2 per unit of energy produced than carbohydrate oxidation (Frayn 1983).
The remainder of the reduction in moderate-to-heavy intensity transition power output was accounted for by decreased rates of metabolic energy expenditure at the transition (Fig. 4d). This is demonstrated by the reduction in rates of energy expenditure as well as V·̇O2 at the moderate-to-heavy intensity transition from PRE to POST (Fig. 4ab). Plausibly, the observed reduction in energy expenditure at the moderate-to-heavy intensity transition with prolonged exercise may also be at least partially attributable to decreased endogenous carbohydrate availability. Specifically, localised glycogen depletion in the intramyofibrillar compartment has been linked to impaired excitation–contraction coupling, manifesting as reduced Ca2+ release from the sarcoplasmic reticulum under neural innervation (Ørtenblad et al. 2013). Therefore, intramyofibrillar glycogen depletion during the prolonged exercise of the current study may plausibly have diminished the function of individual working muscle fibres. Evidence for depletion of endogenous carbohydrate availability is provided by the observed increase in whole-body fat oxidation rates from PRE to POST (Fig. 5), given the autoregulatory nature of muscle glycogen metabolism (Hargreaves et al. 1995). In turn, impaired contractile activity of specific muscle fibres due to intramyofibrillar glycogen depletion may have increased the metabolic burden that a given power output placed on the smaller number of active, fully functional fibres. More specifically, greater burden may have been placed on less oxidative type IIAB and IIB later in the prolonged exercise bout, as evidenced by prior work on fibre type-specific glycogen depletion patterns during prolonged exercise (Vøllestad et al. 1984). These proposed effects of glycogen depletion on the moderate-to-heavy transition may have been exacerbated by the exercise of the present study being conducted after an overnight fast and without carbohydrate intake during exercise, and may plausibly therefore be lessened in training and competition scenarios in which exercise is performed postprandially and with carbohydrate feeding. However, as muscle glycogen depletion, and more specifically compartmental muscle glycogen depletion, was not measured in this study, this mechanism remains speculative and could be interrogated in future work. Additionally, the importance of glycogen availability for durability of the moderate-to-heavy transition could be further explored through repetition of the present protocol with experimental manipulation of pre-exercise glycogen availability through exercise and/or nutrition interventions.
Interestingly, the contribution made by decreased efficiency, but not decreased rates of metabolic energy expenditure at the transition, to prolonged exercise-induced changes in the moderate-to-heavy intensity transition power output was related to the magnitude of PRE to POST changes in whole-body fat oxidation rates; that is, those for whom decreases in energetic efficiency were large exhibited the largest PRE to POST increases in whole-body fat oxidation rates (Table 2). Larger increases in fat oxidation following prolonged exercise may reflect greater muscle glycogen depletion (Hargreaves et al. 1995), and glycogen depletion may as discussed negatively impact contractile function and in turn efficiency at the level of the muscle fibre (Ørtenblad et al. 2013). These data may therefore indirectly support that the loss of energetic efficiency with prolonged exercise was at least partially attributable to the degree of glycogen depletion. However, these data are associational and muscle glycogen was not measured in the present study, and so this mechanism should be interrogated directly in future work.
In contrast, the reduction in moderate-to-heavy intensity transition power output was not significantly associated with PFO (Table 1). The PFO is a marker of an individual’s capacity for fat oxidation during exercise (Maunder et al. 2018), meaning that having a greater capacity to oxidise fatty acids in a fresh state during exercise was not related to ‘durability’ of the moderate-to-heavy intensity transition. If muscle glycogen depletion was the primary mechanism behind the observed prolonged exercise-induced reduction in moderate-to-heavy intensity transition power output, one might have predicted that possessing a greater capacity to oxidise fatty acids during exercise would have mitigated this decline. The absence of a relationship between PFO and the durability of the moderate-to-heavy intensity transition therefore appears to counter this proposed mechanism. However, PFO may not completely reflect the degree of muscle glycogen depletion induced by the prolonged exercise, and therefore to test this hypothesis future work may consider replicating the design of the present study, but with measurements of muscle glycogen content.
As the power output at the moderate-to-heavy intensity transition decreased following acute prolonged exercise, these data suggest that using a well-rested assessment of power output at the moderate-to-heavy intensity transition for programming prolonged exercise risks inadvertent drift from the moderate into the heavy intensity domain. This may have implications for training prescription; specifically, drift into the heavy domain may extend the recovery required after sessions intended to be of moderate intensity and therefore low physiological stress (Seiler et al. 2007; Stanley et al. 2013). Similarly, training load models may need to consider accounting for the durability of intensity domain transitions to better quantify training load.
During the two-hour constant work-rate phase, heart rate increased by 8.2 ± 2.7% from 15 to 120 min (Fig. 2a). This may have been related to increases in core temperature and therefore cutaneous blood flow, progressive dehydration and therefore reduced stroke volume (Coyle and Gonzalez-Alonso 2001), as well as the increased metabolic demand of the fixed work rate (Fig. 2b). More importantly, and in contrast to our hypothesis, it was observed that the heart rate associated with VT1 significantly increased from PRE to POST (6.3 ± 5.8%, Fig. 4c). These data demonstrate that the cardiovascular drift that occurred with acute prolonged exercise was proportionally larger than the downward drift in the power output associated with the moderate-to-heavy intensity transition, and therefore that the heart rate associated with the moderate-to-heavy intensity transition increases over time during acute prolonged exercise. These results therefore suggest use of well-rested assessments of the heart rate at the moderate-to-heavy intensity transition to prescribe prolonged exercise may risk ‘undertraining’, or downward drift within the moderate-intensity domain over time.
In the present study there was inter-individual variation in the degree of reduction in moderate-to-heavy intensity transition power output following prolonged exercise, with the reduction in VT1 power output ranging from ~ 9–44 W (Fig. 3). This suggests that the durability of the moderate-to-heavy intensity transition is not a uniform characteristic between-athletes, and thus that profiling the effects of prolonged exercise at the individual level may be useful for capturing an endurance athlete’s physiological profile. Moderate strength relationships were observed between the initial VT1 power output, expressed as %V·̇O2peak, and the durability of the moderate-to-heavy intensity transition, although this relationship was not present when the moderate-to-heavy intensity transition was estimated using blood lactate data (Table 1). Future work may consider exploring the implications of this characteristic for endurance performance, and also the training-related and physiological characteristics that differentiate athletes with high vs. low durability.
In conclusion, the present investigation demonstrated prolonged moderate-intensity cycling significantly reduced the power output observed at the moderate-to-heavy intensity transition. This reduction was associated with decreased gross cycling efficiency and rates of metabolic energy expenditure at the transition. The heart rate associated with this transition increased following prolonged exercise. Therefore, it may be important for endurance athletes to understand how intensity transitions are affected by prolonged exercise at an individual level to refine physiological profiling, training prescription, and load monitoring.
Data availability
Data are available from the corresponding author upon reasonable request.
Code availability
None used.
Abbreviations
- EE:
-
Energy expenditure
- HR:
-
Heart rate
- PFO:
-
Peak fat oxidation
- \(\dot{V}\)CO2 :
-
Rate of carbon dioxide production
- \(\dot{V}_{E}\) :
-
Rate of ventilation
- \(\dot{V}\)O2 :
-
Rate of oxygen consumption
- \(\dot{V}\)O2peak:
-
Peak rate of oxygen consumption
- VT1 :
-
First ventilatory threshold
References
Burnley M, Jones AM (2018) Power–duration relationship: Physiology, fatigue, and the limits of human performance. Eur J Sport Sci 18:1–12. https://doi.org/10.1080/17461391.2016.1249524
Clark IE, Vanhatalo A, Bailey SJ et al (2018) Effects of two hours of heavy-intensity exercise on the power–duration relationship. Med Sci Sports Exerc 50:1658–1668. https://doi.org/10.1249/MSS.0000000000001601
Clark IE, Vanhatalo A, Thompson C et al (2019a) Dynamics of the power-duration relationship during prolonged endurance exercise and influence of carbohydrate ingestion. J Appl Physiol 127:726–736. https://doi.org/10.1152/japplphysiol.00207.2019
Clark IE, Vanhatalo A, Thompson C et al (2019b) Changes in the power-duration relationship following prolonged exercise: estimation using conventional and all-out protocols and relationship with muscle glycogen. Am J Physiol - Regul Integr Comp Physiol 317:R59–R67. https://doi.org/10.1152/ajpregu.00031.2019
Coyle EF, Gonzalez-Alonso J (2001) Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev 29:88–92
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634
Hargreaves M, McConell G, Proietto J (1995) Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J Appl Physiol 78:288–292
Hopker JG, O’Grady C, Pageaux B (2017) Prolonged constant load cycling exercise is associated with reduced gross efficiency and increased muscle oxygen uptake. Scand J Med Sci Sport 27:408–417. https://doi.org/10.1111/sms.12673
Jamnick NA, Botella J, Pyne DB, Bishop DJ (2018) Manipulating graded exercise test variables affects the validity of the lactate threshold and VO2peak. PLoS ONE 13:1–21. https://doi.org/10.1371/journal.pone.0199794
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26:S28–S37. https://doi.org/10.1055/s-2004-830512
Jones AM, Grassi B, Christensen PM et al (2011) Slow component of VO2 kinetics: mechanistic bases and practical applications. Med Sci Sports Exerc 43:2046–2062
Jones AM, Burnley M, Black MI et al (2019) The maximal metabolic steady state redefining the gold standard. Physiol Rep 7(10):1–16. https://doi.org/10.14814/phy2.14098
Maunder E, Plews DJ, Kilding AE (2018) Contextualising maximal fat oxidation during exercise: determinants and normative values. Front Physiol 9:1–13. https://doi.org/10.3389/fphys.2018.00599
Maunder E, Seiler S, Mildenhall MJ et al (2021) The importance of ‘durability’ in the physiological profiling of endurance athletes. Sports Med 51:1619–1628. https://doi.org/10.1007/s40279-021-01459-0
Maunder E, Plews DJ, Wallis GA et al (2022) Peak fat oxidation is positively associated with vastus lateralis CD36 content, fed-state exercise fat oxidation, and endurance performance in trained males. Eur J Appl Physiol 122:93–102. https://doi.org/10.1007/s00421-021-04820-3
Ørtenblad N, Westerblad H, Nielsen J (2013) Muscle glycogen stores and fatigue. J Physiol 591:4405–4413. https://doi.org/10.1113/jphysiol.2013.251629
Passfield L, Doust JH (2000) Changes in cycling efficiency and performance after endurance exercise. Med Sci Sports Exerc 32:1935–1941. https://doi.org/10.1097/00005768-200011000-00018
Seiler S, Haugen O, Kuffel E (2007) Autonomic recovery after exercise in trained athletes: Intensity and duration effects. Med Sci Sports Exerc 39:1366–1373. https://doi.org/10.1249/mss.0b013e318060f17d
Stanley J, Peake JM, Buchheit M (2013) Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med 43:1259–1277. https://doi.org/10.1007/s40279-013-0083-4
Vøllestad NK, Vaage O, Hermansen L (1984) Muscle glycogen depletion patterns in type I and subgroups of type II fibres during prolonged severe exercise in man. Acta Physiol Scand 122:433–441
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No sources of funding were used in the completion of this study.
Author information
Authors and Affiliations
Contributions
JDS, EM, AEK, and DJP: conceived and designed the research. JDS and EM: conducted experiments and collected the data. JDS and EM: analysed the data. JDS and EM: drafted the manuscript. All authors read, revised, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests associated with this manuscript.
Ethics approval
The Auckland University of Technology Ethics Committee approved all procedures (21/253).
Consent to participate
All participants provided written informed consent.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.





Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stevenson, J.D., Kilding, A.E., Plews, D.J. et al. Prolonged cycling reduces power output at the moderate-to-heavy intensity transition. Eur J Appl Physiol 122, 2673–2682 (2022). https://doi.org/10.1007/s00421-022-05036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-05036-9