Abstract
Background
Humans display an age-related decline in cerebral blood flow and increase in blood pressure (BP), but changes in the underlying control mechanisms across the lifespan are less well understood. We aimed to; (1) examine the impact of age, sex, cardiovascular disease (CVD) risk, and cardio-respiratory fitness on dynamic cerebral autoregulation and cardiac baroreflex sensitivity, and (2) explore the relationships between dynamic cerebral autoregulation (dCA) and cardiac baroreflex sensitivity (cBRS).
Methods
206 participants aged 18–70 years were stratified into age categories. Cerebral blood flow velocity was measured using transcranial Doppler ultrasound. Repeated squat-stand manoeuvres were performed (0.10 Hz), and transfer function analysis was used to assess dCA and cBRS. Multivariable linear regression was used to examine the influence of age, sex, CVD risk, and cardio-respiratory fitness on dCA and cBRS. Linear models determined the relationship between dCA and cBRS.
Results
Age, sex, CVD risk, and cardio-respiratory fitness did not impact dCA normalised gain, phase, or coherence with minimal change in all models (P > 0.05). cBRS gain was attenuated with age when adjusted for sex and CVD risk (young–older; β = − 2.86 P < 0.001) along with cBRS phase (young–older; β = − 0.44, P < 0.001). There was no correlation between dCA normalised gain and phase with either parameter of cBRS.
Conclusion
Ageing was associated with a decreased cBRS, but dCA appears to remain unchanged. Additionally, our data suggest that sex, CVD risk, and cardio-respiratory fitness have little effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing is a non-modifiable risk factor for cerebrovascular diseases (Boehme et al. 2017). Evidence shows that both cerebral blood flow (CBF) (Lu et al. 2011) and cerebral blood flow velocity (CBFv) decline with age (Ainslie et al. 2008). Yet, age-related changes in cerebrovascular function and its interaction with systemic haemodynamic regulation are not well established. Within the cerebrovasculature, the intrinsic ability to maintain adequate CBF in the presence of transient changes in blood pressure (BP) that occur over a number of seconds is referred to as dynamic cerebral autoregulation (dCA) (Aaslid et al. 1989; Claassen et al. 2016). dCA acts as a defensive mechanism protecting the brain from potential damage from high or low BP (van Beek et al. 2008). Simultaneously neural control of systemic BP occurs via the baroreceptors, yet the relationship between these two regulating processes has not been well studied and may provide insightful mechanistic information into CBF regulation. Indeed, whether changes in BP control alter acute cerebral haemodynamics may in turn provide a potential target for interventions.
Previous research assessing dCA using forced BP oscillations with repeated squat-stand manoeuvres has shown, despite age-related reductions in CBFv and increases in BP (Ainslie et al. 2008), there is little evidence of impairment in dCA between groups of young and old (mean age 23 vs 66 years) healthy individuals (Smirl et al. 2015). A finding which has also been replicated within clinical populations (e.g., Alzheimer’s) (Claassen et al. 2009a; Smirl et al. 2014; Lewis et al. 2019). Xing et al. (2017), in a larger sub-sample of individuals across the age range 18–70 years, also observed that dCA from driven oscillations was not different across the lifespan in healthy individuals free of cardiovascular disease (CVD). Although there was some evidence that women had a better dCA compared to men, this finding was in contrast to a recent study (Labrecque et al. 2019a). One research group have also highlighted the potential importance of cardio-respiratory fitness when assessing sex differences in dCA (Labrecque et al. 2017, 2019a, b). Despite this, no study has examined the interaction of sex, cardio-respiratory fitness, or CVD risk factors on dCA in a large sample of individuals across the life span.
Ageing negatively influences cardiac BRS (cBRS) (Monahan 2007), with conflicting evidence as to whether there are sex differences in this response (Xing et al. 2017; Okada et al. 2012), and Hart et al. (2011). cBRS positively correlates with dCA in young but not middle aged or older healthy participants (Xing et al. 2017). However, the fundamental relationship between dCA and cBRS is unclear as other studies suggest an inverse relationship in young healthy individuals (Tzeng et al. 2010), and no relationship in older endurance trained athletes (Aengevaeren et al. 2013) or in heart transplant recipients (Smirl et al. 2014). Understanding such relationships is further complicated by the use of a number of different techniques to bring about changes in BP and analysis methods to quantify dCA and cBRS. Our aim was twofold; (1) to examine the impact of sex, cardio-respiratory fitness, and CVD risk factors on dCA and cBRS over the life span; and (2) to explore the relationships between cBRS and dCA whilst controlling for age and sex. To address these aims, we used secondary data from studies undertaken in our laboratory, that employed the same technique to bring about changes in BP (repeated squat-stand manoeuvres) and analysis method (transfer function analysis) (Claassen et al. 2016), in a large sample of individuals.
Methods
Participants
Data from 11 studies collected at Liverpool John Moores University, Research Institute for Sport and Exercise Science were examined for eligibility. Data were included if: (1) all measurements were performed with strict adherence to Cerebral Autoregulation Network (CARNet) guidelines (Claassen et al. 2016), (2) individual-level minimum dataset was available [i.e., age, sex, body mass index (BMI), and resting BP], and (3) data were collected in studies that adhered to the Declaration of Helsinki. Data were included from four previously published studies (Carter et al. 2018, 2020; Maxwell et al. 2019; Brislane et al. 2020) where dCA and cBRS recordings were collected with corresponding participant characteristics and medical history (where available). When studies adopted a repeated-measures design, only baseline data were included. Participant data was excluded if the duration of recordings was < 5-min, and if the coherence value was < 0.4 (Claassen et al. 2016). Based on these criteria, 206 participants were included consisting 83 males and 123 females aged between 18 and 70 years. All participants were non-smokers, with no previous myocardial infarction, stroke, or thrombosis. Individuals clinically diagnosed with Type 2 diabetes mellitus (T2DM) were treated with Metformin (n = 18) or diet (n = 8) at the time of data collection. Additional medications taken by participants included anti-hypertensive (n = 15) and lipid lowering (n = 16) medication. Participants that had a BMI > 30 kg/m2, diagnosed with hypercholesterolemia or T2DM, as well treated or untreated ≥ stage 1 hypertension were stratified to a CVD risk group. Fifty eight of the females were post-menopausal. These women were classified based on having no menstrual cycle for at least 12 consecutive months and not previously or currently taking any form of hormone therapy (Moreau et al. 2012).
Protocol
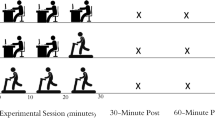
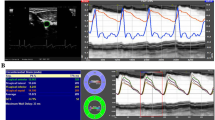
All participants arrived at the laboratory following an overnight fast and had refrained from alcohol and exercise for ≥ 24 h, and caffeine for ≥ 12 h, prior to the visit. Following a minimum of 20 min supine rest, measurements of middle cerebral artery velocity (MCAv) were obtained using transcranial Doppler ultrasound (TCD) following standardised procedures (Willie et al. 2011). Two 2-MHz Doppler probes (Spencer Technologies, Seattle, USA) were placed over the temporal window and adjusted until an optimal signal was identified and held in place using a Marc 600 head frame (Spencer Technologies, Seattle, USA). Beat-to-beat blood pressure was recorded using a Finometer (Model 1, Finapres Medical Systems BV, Amsterdam, The Netherlands). Participants were fitted with a photoplethysmographic cuff on the right index finger, and the output was corrected by referencing the cuff to heart level using a height correction unit and heart rate (HR) acquired from a 3-lead electrocardiogram. Partial pressure of end tidal carbon dioxide (PETCO2) was continuously monitored by instrumenting participants with a two-way valve mouthpiece (Hans Rudolph) connected to a calibrated gas analyser (ML206 ADinstruments, Colorado Springs, USA). All data were sampled at 50 Hz with the data acquisition system PowerLab via the interface LabChart 7 (ADinstruments, Colorado Springs, USA).
Baseline haemodynamics
Resting MCAv, HR, mean arterial pressure (MAP) and PETCO2 were continuously recorded for 5 min in the supine position. Participants were instructed to maintain normal breathing and refrain from closing their eyes. Baseline data were averaged over the 5-min recording.
Dynamic cerebral autoregulation
dCA was assessed using repeated squat-stand manoeuvres to induce oscillations in MAP. This technique has been shown to be the best protocol for eliciting high interpretable linearity between MAP and MCAv signals (Smirl et al. 2015; Claassen et al. 2009b). Beginning in a standing position, the participants mimic the experimenter by squatting down obtaining a ≈ 90° angle and then returning to the standing position. The manoeuvres were performed at a frequency of 0.10 Hz (5-s squat–5-s stand) for a period of 5 min. This frequency of manoeuvre was performed as these large oscillations in MAP are extensively buffered by cerebral vessels when completed at frequencies within the high-pass filter buffering range (< 0.20 Hz) (Zhang et al. 1998). By executing repeated squat-stand manoeuvres, this optimises the signal-to-noise ratio and improves the interpretability of the recordings through the physiologically relevant change in MAP (Smirl et al. 2015). Whilst performing the manoeuvres, participants were instructed to maintain normal breathing and avoid Valsalva manoeuvres. Throughout the 5-min protocol, MCAv, HR, MAP, and PETCO2 were continuously assessed.
Data for dCA were analysed in accordance with the most recent recommendations from the CARNet (Claassen et al. 2016). Both beat-to-beat MCAv and MAP signals were extracted from LabChart and then spline interpolated before being re-sampled at 4 Hz for spectral analysis and transfer function analysis (TFA) based on the Welch algorithm. Each of the 5-min recordings was subdivided into 5 successive windows overlapping by 50%. Each window was passed through a Hanning window prior to Fourier transformation. The cross spectrum between MCAv and MAP was determined for TFA by the MAP auto-spectrum to determine transfer function parameters absolute gain, normalised gain, phase, and coherence. dCA data (squat–stand manoeuvres) were sampled at the point estimate of the driven frequency (0.10 Hz). TFA parameters were only included for subsequent analysis when coherence exceeded 0.4. Additionally, data were excluded if 5 min of clear artifact free recordings were not present.
Cardiac baroreflex sensitivity
During the same 5-min 0.10 Hz squats-stand manoeuvres, continuous cBRS was measured. The cBRS was determined by applying TFA to systolic BP and R–R interval (pressure-cardiac interval) at the point estimate of the driven frequency of the squat-stand manoeuvres (0.10 Hz). Data analysis was performed using a commercially available software Ensemble (Version 1.0.0.28, Elucimed, Wellington, New Zealand). Mean gain, phase, and coherence along with spectral power of systolic BP and R–R interval were calculated in the low-frequency range.
Cardio-respiratory fitness
Breath-by-breath expired gases were continuously monitored (Oxycon Pro, Jaeger, Hochberg Germany) for oxygen consumption (ml/kg/min) during an incremental maximal exercise test and were averaged over 15 s (Sprung et al. 2013). Peak oxygen uptake was calculated from the highest consecutive 15-s period of expired gas fractions. All participants reached the criteria for volitional exhaustion based upon heart rate, peak oxygen uptake, Borg scale, and respiratory exchange ratio (Sprung et al. 2013; Bailey et al. 2016).
Statistical analysis
Statistical analysis was performed using IBM SPSS version 26 (SPSS Inc., Chicago, IL). First, participants were stratified into three age categories: young (18–35 years, n = 93), middle age (36–55 years, n = 62), and old age (56–70 years, n = 51). Between age-category differences in baseline characteristics and power spectrum densities during squat-stand manoeuvres were explored using one-way ANOVA. To examine the influence of age, sex, CVD risk, and VO2max linear regression was employed. Cross-sectional associations between age and measures of dCA and cBRS were examined using linear regression adjusting for sex (Model 1). Multivariable linear regression was used to further adjust for health status (model 2) as well as VO2max (model 3). To examine specifically changes associated with cBRS and menopause, pre vs post-menopausal women were compared using a general linear model with age as covariate.
Relationship between cardiac BRS and dCA
The linear relationship between cBRS and dCA was determined using the Coefficient of determination (R2). For the models, each parameter of cBRS was independently used as a predictor variable and each parameter of dCA an outcome variable with adjustments for age and sex. Evidence of multicollinearity was explored using the variance inflation factor. Statistical significance was set a P < 0.05.
Results
Participant characteristics
There was an increase in SBP, DBP, and BMI (P < 0.001) and decrease in MCAv and VO2max (P < 0.001) with age (Table 1) at baseline. Age, SBP, DBP, and BMI were significantly higher (P < 0.001) in the CVD risk group compared to healthy, with VO2max and MCAv significantly lower in the CVD risk group (P < 0.001) (Table 1).
dCA
Age, sex, CVD risk factors, and VO2max did not impact dCA parameters normalised gain, phase or coherence with minimal change (β) compared to the young aged reference group (18–35 years) in all statistical models (P > 0.05, Table 2). There was a significant reduction in dCA gain with age, which was apparent when adjusted for sex and CVD risk factors (young—middle age; β = − 0.09, P = 0.02 and young—old age; β = − 0.18, P < 0.001, model 2) but not when adjusted for VO2max (model 3).
BRS
cBRS gain was attenuated with age when adjusted for sex and CVD risk factors (young—middle age; β = − 2.18, P < 0.001 and young—old age; β = − 2.86 P < 0.001, model 2) along with BRS phase (young—middle age; β = − 0.31, P < 0.001 and young—old age; β = − 0.44 P < 0.001, model 2) but not adjusted for VO2max (model 3). cBRS gain was significantly lower in the post-menopausal group compared to pre-menopausal (− 1.59 ms/mmHg; 95% CI − 2.43–0.77 P < 0.001) but not when using age as a covariate (− 0.50 ms/mmHg; 95% CI − 2.06, 1.06 P = 0.79). Similarly, cBRS phase was attenuated in the post-menopausal group compared to pre-menopausal (− 0.39 radians; 95% CI − 0.62, 0.15 P = 0.02) but not when using age as a covariate (0.06 radians; 95% CI − 0.36, 0.45 P = 0.77).
Power spectral analysis
When stratified by age, dCA BP power, MCAv power, and cardiac BRS R–R interval power all demonstrated a negative relationship (P < 0.001) with no difference in SBP power (P = 0.55, Table 3).
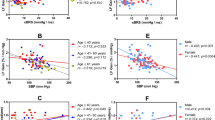
Relationship between cBRS and dCA
There was little correlation between dCA normalised gain and dCA phase with either parameter of cBRS (P > 0.05; Fig. 1). dCA gain was correlated with cBRS gain (R2 = 0.19, P < 0.001) and with cBRS phase (R2 = 0.18, P < 0.001). However, the total variance explained in these significant outcomes is small, meaning that other factors are likely to be important, whether independent or as interacting variables.
Discussion
The aims of the current study were to (1) examine the impact of sex, cardio-respiratory fitness and the presence of CVD risk factors on dCA and cBRS over the life span; and (2) explore the relationships between cBRS and dCA whilst controlling for age and sex. We present the following observations. First, dCA measured using repeated squat-stand manoeuvres is preserved across the age range of 18–70 years in healthy individuals ex, fitness or the presence of CVD risk factors had little effect. Second, cBRS declined with ageing. Finally, cBRS gain and phase displayed no relationship with dCA.
Ageing is a risk factor for cerebrovascular disease and complications. A number of cerebral haemodynamic parameters change with age, including reductions in CBF volume and CBFv (Krejza et al. 1999; Ainslie et al. 2008; Lu et al. 2011). Yet, our current data show that the intrinsic ability of cerebral vessels to maintain stable flow in response to acute changes in BP is unaffected by ageing across the lifespan up to the age of 70 years. This suggests that the age-related decline in CBFv is not merely a result of impaired dCA. The ability of the cerebrovasculature to buffer transient changes in BP represents a vital defence mechanism protecting the brain from hypo- and hyperfusion (Claassen and Zhang 2011). Our data are in agreement with the previous studies, with smaller sample sizes or age group comparisons, which identified no reduction in dCA with older age using both squat-stand manoeuvres (Xing et al. 2017; Smirl et al. 2014; Oudegeest-Sander et al. 2014) or other dCA techniques (Yam et al. 2005; Carey et al. 2000; Dineen et al. 2011). We also show that dCA is not different between sexes when age is considered. Whilst some previous work has identified interactions between sex and dCA (Deegan et al. 2011; Labrecque et al. 2019a), suggesting this as possible explanation for increased orthostatic hypotension-related complications, the data from our large sample study did not show any interactions between sex across age ranges.
Another novel aspect of our study was that we examined the impact of the presence of CVD risk factors on the decline in dCA. Central obesity, hypertension, hypercholesterolemia, and T2DM represent major risk factors in the development of systemic vascular disease and complications (Seven 2015) including significantly increased risk of cerebrovascular disease (Law et al. 2009; Kivipelto et al. 2005; Pinto et al. 2004). Each risk factor individually or collectively is associated with endothelial dysfunction, increased arterial stiffness, alongside a range of other vascular abnormalities (Stapleton et al. 2008). Despite these vascular changes, none of these CVD risk factors included within our study were associated with a reduction in dCA when age in considered. Our group has previously shown that in a small sample of individuals with increased CVD risk, dCA is not different to that of young healthy individuals (Carter et al. 2020); with the current study, we confirm the original observation using a markedly larger sample size. To date, no other studies have assessed dCA using squat-stand manoeuvres in a population with these specific risk factors for CVD. Comparisons between previous studies that have assessed cerebral autoregulation in similar cohorts are challenging because of methodological differences. Previous studies employing squat-stand maneuverers and TFA examined one CVD risk factor, i.e., hypertension (Lipsitz et al. 2000; Eames et al. 2003) and T2DM (Huq et al. 2012) and also observed no change in dCA. Studies utilising the exact same dCA methods used in our study have observed no difference in patients with chronic obstructive lung disease (Lewis et al. 2019), in early stage Alzheimer’s (Claassen et al. 2009a), or even in heart transplant recipient patients (Smirl et al. 2014). Collectively, our data suggest that despite the vascular maladaptations that are associated with CVD risk factors, the intrinsic ability of the cerebral blood vessels to maintain stable flow upon fluctuations in BP is persevered.
Elevated cardio-respiratory fitness is associated with increased resting CBFv values (Ainslie et al. 2008) and enhanced cerebrovascular reactivity (Bailey et al. 2013), but its association with dCA is less clear. In fact, cardio-respiratory fitness may be important when assessing dCA (Labrecque et al. 2019b). Using a relatively large sample size, of moderately fit individuals, we found VO2max is not related to variations in dCA. Interestingly, two previous studies concluded that higher VO2max was related to attenuated dCA (Labrecque et al. 2017; Lind-Holst et al. 2011), whereas Aengevaeren et al. (2013) identified no effect of VO2max on dCA. Disparities in the study findings are likely due to differences in dCA assessment methods, but could also be explained by including individuals with fitness levels at the lower and higher ends of the continuum. Moreover, differences in specific training status may alter dCA responses independent of VO2max; for example, the work by Labrecque et al. (2017) recruited individuals with a training load of 12 h per week for a minimum of 2 years, whereas in our study, we did not take into consideration training load/status but rather just cardio-respiratory fitness based on a maximum capacity exercise test. Whether any changes associated with improved/reduction in dCA are directly related to cardio-respiratory fitness or vascular/neural adaptations to chronic exercise requires further investigation. Our data provide some evidence, in a demographically varied cohort, using a single method of dCA assessment with TFA, suggesting that VO2max has little impact on dCA, albeit within a small range of moderately fit individuals.
Our data further support a wealth of research that shows cBRS declines with age (Monahan 2007; Xing et al. 2017; Smirl et al. 2014; O'Mahony et al. 2000; La Rovere et al. 2008). We provide evidence that CVD risk factors are linked to reduced cBRS (Skrapari et al. 2007; Sakamoto et al. 2019; Madden et al. 2010) and cBRS across a broad age range in females is reduced in post-menopausal women compared to pre-menopausal (Barnes et al. 2012). We provide some evidence that this is could be explained by age, rather than the menopause accelerating any decline in cBRS. We acknowledge that further investigation is warranted to explore the impact of the menopause. The direct relationship between dCA and cBRS is complex. Understanding whether enhanced BP control leads to better control of CBF or vice visa is important in understanding how these regulatory mechanisms operate, and whether they should be the focus of interventions (Favre and Serrador 2019).
Our study provides evidence that cBRS parameters show no relationship with dCA normalised gain and dCA phase during forced BP oscillations, but do appear to have a relationship with dCA (absolute) gain. Absolute gain reflects absolute CBFv changes (Claassen et al. 2016), and thus with both CBFv and cBRS reducing with age, it is likely to result in a significant association between the two parameters, but when dCA gain is normalised for changes in BP no relationship is present. Interestingly, one previous study using TCD to measure rate of regulation and autoregulation index for dCA and the modified Oxford technique to estimate BRS, reported an inverse relationship between the two processes (Tzeng et al. 2010). This implies that the lower an individual’s BRS (i.e., reduced BP control), the more effective their dCA is at counteracting large fluctuations in BP and could imply an increased efficiency of dCA in protecting against the age-related decline in cBRS and various haemodynamic changes. On the other hand, previous studies utilising the same methods adopted in this present study concluded no relationship between dCA and cBRS parameters (Smirl et al. 2014; Aengevaeren et al. 2013). Therefore, the data from our study outline that despite having a significantly greater BP control at a younger age, this does not alter how well the cerebral vessels regulate blood flow during BP challenges. Differences in study findings may simply be explained the assessment of cBRS and dCA, as work by Horsman et al. (2014) demonstrated that in squat-stand manoeuvres at 0.10 Hz hysteresis is present which was not with the modified oxford technique. The squat-stand manoeuvres produce significant fluctuations in central blood volume and total peripheral resistance The overall input of the baroreceptors and neural feedback on dCA is complex and not well understood (Ainslie and Brassard 2014) with evidence from animal studies, showing that isolated dual elimination of baroceptor and chemoreceptor completely abolished cerebral autoregulation in dogs, whereas cerebral autoregulation was preserved in in sympathetically and parasympathetically denervated animals (Sagawa and Guyton 1961; Busija and Heistad 1984).
Our study utilises a large sample size, in a demographically varied cohort, employing the same technique and adhering to published guidelines. However, we acknowledge a number of limitations. First, the use of TCD assesses blood flow velocity rather than blood flow as arterial diameter is not taken into consideration and therefore a stable diameter cannot be verified. MCA diameter has been shown to be consistent during modest changes in CO2 (± 5 mmHg) (Ainslie and Hoiland 2014), as well as acute moderate changes in BP (Giller et al. 1993; Serrador et al. 2000); thus, our data should be interpreted with some caution. Second, we employed 0.10 Hz squat-stand manoeuvres only to interrogate dCA, incorporating 0.05 Hz manoeuvres and spontaneous oscillations may have provided an additional level of detail and strengthened interpretability of the results. Third, ageing was used as an individual variable within our analysis rather than incorporating it into the CVD risk factors section, as age does represent a major non-modifiable risk factor for CVD. Finally, the Bruce protocol was utilised for the assessment of VO2max and using this protocol may have resulted in an underestimation of the VO2max in the younger individuals.
In conclusion, we show that older age was associated with a decreased baroreflex sensitivity, but dCA appears to remain stable with ageing, sex, CVD risk, and cardio-respiratory fitness have little effect. Therefore, cerebral vessels regulate blood flow during acute BP challenges across the age span.
Data availability
The data that support the findings of this study are available from the corresponding author upon a reasonable request.
Code availability
Not applicable.
References
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H (1989) Cerebral autoregulation dynamics in humans. Stroke 20:45–52
Aengevaeren VL, Claassen JA, Levine BD, Zhang R (2013) Cardiac baroreflex function and dynamic cerebral autoregulation in elderly Masters athletes. J Appl Physiol 1985(114):195–202
Ainslie PN, Brassard P (2014) Why is the neural control of cerebral autoregulation so controversial? F1000prime Rep 6:14–14
Ainslie PN, Hoiland RL (2014) Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol 1985(117):1081–1083
Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G (2008) Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586:4005–4010
Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN (2013) Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44:3235–3238
Bailey TG, Cable NT, Miller GD, Sprung VS, Low DA, Jones H (2016) Repeated warm water immersion induces similar cerebrovascular adaptations to 8 weeks of moderate-intensity exercise training in females. Int J Sports Med 37:757–765
Barnes JN, Matzek LJ, Charkoudian N, Joyner MJ, Curry TB, Hart EC (2012) Association of cardiac baroreflex sensitivity with blood pressure transients: influence of sex and menopausal status. Front Physiol 3:187–187
Boehme AK, Esenwa C, Elkind MS (2017) Stroke risk factors, genetics, and prevention. Circ Res 120:472–495
Brislane Á, Low DA, Carter SE, Holder SM, Jones H, Hopkins ND (2020) Cerebral and peripheral vascular differences between pre- and postmenopausal women. Menopause 27:170–182
Busija DW, Heistad DD (1984) Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol 101:161–211
Carey BJ, Eames PJ, Blake MJ, Panerai RB, Potter JF (2000) Dynamic cerebral autoregulation is unaffected by aging. Stroke 31:2895–2900
Carter SE, Draijer R, Holder SM, Brown L, Thijssen DHJ, Hopkins ND (2018) Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol 125:790–798
Carter HH, Maxwell JD, Hellsten Y, Thompson A, Thijssen DHJ, Jones H (2020) The impact of acute remote ischaemic preconditioning on cerebrovascular function. Eur J Appl Physiol 120:603–612
Claassen JA, Zhang R (2011) Cerebral autoregulation in Alzheimer’s disease. J Cereb Blood Flow Metab 31:1572–1577
Claassen JA, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R (2009a) Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using transcranial Doppler. J Alzheimers Dis 17:621–629
Claassen JA, Levine BD, Zhang R (2009b) Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol 1985(106):153–160
Claassen JA, Meel-VAN Den Abeelen AS, Simpson DM, Panerai RB (2016) Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab 36:665–680
Deegan BM, Sorond FA, Galica A, Lipsitz LA, O’laighin, G. & Serrador, J. M. (2011) Elderly women regulate brain blood flow better than men do. Stroke 42:1988–1993
Dineen NE, Panerai RB, Brodie F, Robinson TG (2011) Effects of ageing on cerebral haemodynamics assessed during respiratory manoeuvres. Age Ageing 40:199–204
Eames P, Eames PJ, Blake MJ, Panerai RB, Potter JF (2003) Cerebral autoregulation indices are unimpaired by hypertension in middle aged and older people*. Am J Hypertens 16:746–753
Favre ME, Serrador JM (2019) Reply to “On the need of considering cardiorespiratory fitness when examining the influence of sex on dynamic cerebral autoregulation.” Am J Physiol Heart Circ Physiol 316:H1230-h1231
Giller CA, Bowman G, Dyer H, Mootz L, Krippner W (1993) Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32:737–742
Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, CHARKOUDIAN, N. (2011) Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J Physiol 589:3395–3404
Horsman HM, Tzeng YC, Galletly DC, Peebles KC (2014) The repeated sit-to-stand maneuver is a superior method for cardiac baroreflex assessment: a comparison with the modified Oxford method and Valsalva maneuver. Am J Physiol-Regulat Integr Compar Physiol 307:R1345–R1352
Huq R, Philbey CE, Mistri AK, Panerai RB, Robinson TG (2012) Dynamic cerebral autoregulation assessed by respiratory manoeuvres in non-insulin-treated Type 2 diabetes mellitus. Diabet Med 29:609–613
Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, Helkala E-L, Tuomilehto J, Soininen H, Nissinen A (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. JAMA Neurol 62:1556–1560
Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A (1999) Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol 172:213–218
La Rovere MT, Pinna GD, Raczak G (2008) Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13:191–207
Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Drapeau A, Smirl JD, Bailey DM, Brassard P (2019a) Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep 7:e13984
Labrecque L, Smirl JD, Brassard P (2019b) Letter to the Editor: On the need of considering cardiorespiratory fitness when examining the influence of sex on dynamic cerebral autoregulation. Am J Physiol-Heart Circulat Physiol 316:H1229–H1229
Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Lucas SJE, Bailey DM, Smirl JD, Brassard P (2017) Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiol Rep 5:e13486
Law MR, Morris JK, Wald NJ (2009) Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338:b1665
Lewis N, Gelinas JCM, Ainslie PN, Smirl JD, Agar G, Melzer B, Rolf JD, Eves ND (2019) Cerebrovascular function in patients with chronic obstructive pulmonary disease: the impact of exercise training. Am J Physiol-Heart Circulat Physiol 316:H380–H391
Lind-Holst M, Cotter JD, Helge JW, Boushel R, Augustesen H, van Lieshout JJ, Pott FC (2011) Cerebral autoregulation dynamics in endurance-trained individuals. J Appl Physiol 110:1327–1333
Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V (2000) Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke 31:1897–1903
Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC (2011) Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 21:1426–1434
Madden KM, Lockhart C, Potter TF, Cuff D (2010) Aerobic training restores arterial baroreflex sensitivity in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Clin J Sport Med 20:312–317
Maxwell JD, Carter HH, Hellsten Y, Miller GD, Sprung VS, Cuthbertson DJ, Thijssen DHJ, Jones H (2019) Seven day remote ischaemic preconditioning improves endothelial function in patients with type 2 diabetes mellitus: a randomised pilot study. Eur J Endocrinol 181:659–669
Monahan KD (2007) Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293:R3–R12
Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM (2012) Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97:4692–4700
Okada Y, Galbreath MM, Shibata S, Jarvis SS, Vangundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q (2012) Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension (dallas, Tex.: 1979) 59:98–104
O’Mahony D, Bennett C, Green A, Sinclair AJ (2000) Reduced baroreflex sensitivity in elderly humans is not due to efferent autonomic dysfunction. Clin Sci (lond) 98:103–110
Oudegeest-Sander MH, Van Beek AH, Abbink K, Olde Rikkert MG, Hopman MT, Claassen JA (2014) Assessment of dynamic cerebral autoregulation and cerebrovascular CO2 reactivity in ageing by measurements of cerebral blood flow and cortical oxygenation. Exp Physiol 99:586–598
Pinto A, Tuttolomondo A, di Raimondo D, Fernandez P, Licata G (2004) Cerebrovascular risk factors and clinical classification of strokes. Semin Vasc Med 4:287–303
Sagawa K, Guyton AC (1961) Pressure-flow relationships in isolated canine cerebral circulation. Am J Physiol 200:711–714
Sakamoto M, Matsutani D, Kayama Y (2019) Clinical implications of baroreflex sensitivity in type 2 diabetes. Int Heart J 60:241–246
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31:1672–1678
Seven E (2015) Overweight, hypertension and cardiovascular disease: focus on adipocytokines, insulin, weight changes and natriuretic peptides. Dan Med J 62:B5163
Skrapari I, Tentolouris N, Perrea D, Bakoyiannis C, Papazafiropoulou A, Katsilambros N (2007) Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (silver Spring) 15:1685–1693
Smirl JD, Haykowsky MJ, Nelson MD, Tzeng YC, Marsden KR, Jones H, Ainslie PN (2014) Relationship between cerebral blood flow and blood pressure in long-term heart transplant recipients. Hypertension 64:1314–1320
Smirl JD, Hoffman K, Tzeng YC, Hansen A, Ainslie PN (2015) Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol 1985(119):487–501
Sprung VS, Cuthbertson DJ, Pugh CJ, Daousi C, Atkinson G, Aziz NF, Kemp GJ, Green DJ, Cable NT, Jones H (2013) Nitric oxide-mediated cutaneous microvascular function is impaired in polycystic ovary sydrome but can be improved by exercise training. J Physiol 591:1475–1487
Stapleton PA, James ME, Goodwill AG, Frisbee JC (2008) Obesity and vascular dysfunction. Pathophysiology 15:79–89
Tzeng YC, Lucas SJ, Atkinson G, Willie CK, Ainslie PN (2010) Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol 1985(108):1162–1168
van Beek AH, Claassen JA, Rikkert MG, Jansen RW (2008) Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab 28:1071–1085
Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN (2011) Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196:221–237
Xing CY, Tarumi T, Meijers RL, Turner M, Repshas J, Xiong L, Ding K, Vongpatanasin W, Yuan LJ, Zhang R (2017) Arterial pressure, heart rate, and cerebral hemodynamics across the adult life span. Hypertension 69:712–720
Yam AT, Lang EW, Lagopoulos J, Yip K, Griffith J, Mudaliar Y, Dorsch NWC (2005) Cerebral autoregulation and ageing. J Clin Neurosci 12:643–646
Zhang R, Zuckerman JH, Giller CA, Levine BD (1998) Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol 274:H233–H241
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JDM, HHC, DHJT, and HJ were involved in the conception or design of the work. Data acquisition and data reduction were performed by JDM, DJB, AB, SEC, GDM, KAR, and HHC. Statistical analysis was performed by JDM and AT. JDM, NDH, DAL AT, JAHR, DHJT, and HJ interpretated the data and drafting the document. All authors critically revised the document for important intellectual content. All authors confirm that they (1) approved the final version of the manuscript, (2) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and (3) all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Ellen Adele Dawson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maxwell, J.D., Bannell, D.J., Brislane, A. et al. The impact of age, sex, cardio-respiratory fitness, and cardiovascular disease risk on dynamic cerebral autoregulation and baroreflex sensitivity. Eur J Appl Physiol 122, 1531–1541 (2022). https://doi.org/10.1007/s00421-022-04933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04933-3