Abstract
Purpose
The role of splenic emptying in O2 transport during aerobic exercise still remains a matter of debate. Our study compared the differences in spleen volume changes between aerobically trained and untrained individuals during step-transition supine cycling exercise at moderate-intensity. We also examined the relationship between spleen volume changes, erythrocyte release, and O2 uptake parameters.
Methods
Fourteen healthy men completed all study procedures, including a detailed medical examination, supine maximal O2 uptake (\({\dot{\text{V}}\text{O}}_{2}\) max.) test, and three step-transitions from 20 W to a moderate-intensity power output, equivalent to \({\dot{\text{V}}\text{O}}_{2}\) uptake at 90% gas exchange threshold. During these step-transitions pulmonary \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), near-infrared spectroscopy of the vastus lateralis, and cardiovascular responses were continuously measured. In parallel, minute-by-minute ultrasonic measurements of the spleen were performed. Blood samples were taken before and immediately after step-transition cycling.
Results
On average, \({\dot{\text{V}}\text{O}}_{2}\) max. was 10 mL kg min−1 (p = 0.001) higher in trained compared to their aerobically untrained peers. In response to supine step-transition cycling, the splenic volume was significantly reduced, and the largest reduction (~ 106 to 115 mL, ~ 38%, p = 0.001) was similar in both aerobically trained and untrained individuals. Erythrocyte concentration and platelet count transiently increased after exercise cessation, with no differences observed between groups. However, the vastus lateralis deoxygenation amplitude was 30% (p = 0.001) greater in trained compared to untrained individuals. No associations existed between: (i) spleen volumes at rest (ii) spleen volume changes (%), (iii) resting hematocrit and oxygen uptake parameters.
Conclusion
Greater splenic emptying and subsequent erythrocyte release do not lead to a slower \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), regardless of individual \({\dot{\text{V}}\text{O}}_{2}\) max. readings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In healthy humans, the spleen plays an important role in oxygen transport during apnea (Bakovic et al. 2003; Bouten et al. 2019; Palada et al. 2007), and exposure to high altitude (Holmström et al. 2021a), primarily due to its ability to contract and release additional red blood cells (RBC’s) into the systemic circulation (Espersen et al. 2002). Early work by Allsop et al. (1992) showed that after a brief period of exercise, the RBC exit the spleen within ~ 60 s, and the spleen emptying transiently increases RBC concentrations in the systemic circulation. In trained divers and healthy controls, Bakovic and co-authors (2003) reported that splenic contraction(s) contributed to prolongation of successive, repeated dry apneas, mainly, because apnea-induced splenic emptying increased the circulating RBC pool. This gave the second and subsequent apneas an advantage over the first one, primarily due to greater O2 storage capacity of the blood. Interestingly, this was not observed in splenectomized individuals (Bakovic et al. 2003). Notably, compelling evidence that splenic emptying also enhances aerobic performance in humans is still missing, despite recent letters to the editor by Schagatay and Holmström (2021) and Engan et al. (2021), suggesting that splenic emptying additionally facilitates O2 transport to active tissues (via RBC release), under all circumstances, including aerobic exercise. Previous studies examining changes in spleen volume via ultrasound imaging during upright aerobic exercise were limited to pre/post data collection protocols (Sperlich et al. 2015; Lodin-Sundström et al. 2021), and the rate of splenic volume reduction remains unknown. This may be due to the challenges associated with accurate quantification of the spleen volume changes during upright cycling, compared to diving simulations. Briefly, during aerobic exercise in the upright position, a clear image of the contracting spleen is sometimes difficult to obtain, as the anatomical position of the spleen implies isolated views in the region of interest, and possibly overlap with other organs. Thus, the role of the spleen in O2 transport during aerobic exercise has not yet been studied in detail.
Recently, we used the step-transition supine cycling model at 90% gas exchange threshold (GET), while measuring spleen volume changes each minute in parallel. This experimental model allowed us to position the ultrasound probe (constantly) at the region of interest, and evade certain methodological and technical drawbacks associated with the ultrasonic imaging of the spleen during upright exercise. Accordingly, a clear image of the contracting spleen was obtained throughout the experimental procedure. We found that splenic volume was reduced by 36%, while greater splenic emptying was not correlated with faster O2 kinetics, independent of the subsequent RBC release (Zubac et al. 2021a). Nevertheless, the role of splenic emptying in O2 transport during aerobic exercise remains a controversial topic. Only ten participants were included in the latter brief study report and the strength of the observed correlation could vary across the cardiorespiratory fitness spectrum and resting spleen volume of the participants. Previous work showed that resting spleen volume was positively correlated with prolonged apnea duration (~ 30% of shared variance) (Schagatay et al. 2012; Elia et al. 2021), and that the spleen volume is increased by ~ 24% after 8 weeks of static apnea training (Bouten et al. 2019). However, it is not clear from the latter studies whether splenic plasticity (adaptation) is due to extensive experience in apnea performance or to individual genetic predispositions (Ilardo et al. 2018).
The current literature predominantly highlighted greater splenic emptying as an advantage of trained individuals over healthy active controls, likely due to their extensive training background. Briefly, Bakovic et al. (2003) found that the magnitude of splenic volume reduction (i.e., splenic emptying) was greater in apnea divers, compared to healthy controls (18% vs 14%, p = 0.001). Similar results, albeit under different experimental conditions, have been reported by others (Hurford et al. 1990; Prommer et al. 2007; Schagatay et al. 2020). For example, Prommer and co-authors (2007) showed that five repeated apneic episodes performed in a heated pool at a depth of 4 m resulted in a 25% reduction in spleen size volume of trained apnea divers, but not in healthy active controls. During a Mt. Everest expedition, at Katmandu camp (at 1370 m), Schagatay et al. (2020) reported that transient spleen volume reduction was twofold greater in trekkers compared to climbers after five maximal apnea(s). Nonetheless, this still remains a matter of debate. Recent work by Elia et al. (2021) found no differences among elite breath hold divers, non-divers, and control subjects in the post-apneic splenic volume reduction or the amount of the RBC’s released during static and dynamic apnea bouts. Thus, in addition to individual differences among the studied populations, the discrepancy between studies could be due to differences in methodological approaches (e.g., data collection protocols), including the time-interval between cessation of exercise/apnea, initiation of the ultrasonic imaging, and blood collection. For example, Prommer et al. (2007) assessed splenic volume reduction and circulating RBC release ~ 3 min. after participants exited the water and removed their neoprene suits, and reported no changes in RBC concentration after repeated apneas. On the other hand, previous work has shown that the spleen contracts immediately after 3 s of breath-hold (Palada et al. 2007), and the time-frame required for spleen volume recovery ranges from 3 to 10 min (Palada et al. 2007; Bakovic et al. 2012; Bouten et al. 2019). Thus, Prommer and co-authors might had missed out on some crucial information regarding the magnitudes and profile of both splenic contractility and reactivity, and thereby underestimating splenic emptying capacity, erythrocyte release and their impact on oxygen utilization.
To extend the current knowledge of splenic emptying during aerobic exercise, we used the recently established supine cycling model. This model allows researchers to obtain a clear picture of the contracting spleen throughout the course of the experiment, and provides a unique opportunity to capture the acute adaptation of the spleen size at a given exercise intensity domain. However, the pulmonary oxygen uptake kinetics (\({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\)) of young healthy individuals during step-transition cycling in the upright and likely supine position(s) at moderate-intensity domain, are constrained by mitochondrial dynamics, rather than oxygen delivery (Rossiter 2011; Poole and Jones 2011). Furthermore, supine on-transient cycling is characterized by slower \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) uptake kinetics, and greater deoxygenation amplitude of knee extensors (Goulding et al. 2020a, b, 2021). Therefore, our study design included near-infrared spectroscopy (NIRS) measurements at the site of vastus lateralis (VL) muscle to gain a more comprehensive insight into local O2 demand, although the underlying mechanisms of skeletal muscle bioenergetics during supine cycling at moderate and heavy intensity domains have been described elsewhere (Gouding et al. 2020, 2021). Here, we reasoned that the reduction in splenic volume during supine cycling would be clearly observed, while the on-going debate in the literature regarding the role of the spleen in oxygen transport during aerobic exercise warrants further investigation. Recent work by Holmström and co-authors (2021b) suggested that apnea-induced splenic contraction (reduction in splenic volume by ~ 46) leads to a transient increase in Hb concentration (by 4.5%), and thereby arguments O2 uptake in endurance trained athletes, but not in sedentary controls. This, however, contradicts recently published short report, in which we found no correlation between \({\dot{\text{V}}\text{O}}_{2}\) max. and splenic volume reduction, while a parallel 3% increase in hemoglobin and hematocrit was observed following moderate-intensity exercise cessation in male participants of varying cardiorespiratory fitness levels. Thus, the objective of the present study was to compare the differences in splenic volume changes between aerobically trained and untrained individuals during a step-transition supine cycling exercise in the moderate-intensity domain. Next, we aimed to examine the relationship between splenic volume changes, subsequent RBC release, and \({\dot{\text{V}}\text{O}}_{2}\) uptake parameters. We hypothesized that the magnitude of the spleen volume reduction would be greater in aerobically trained individuals, compared to aerobically untrained, leading to a greater mobilization of additional RBC, resulting in the increase of the O2 content in the mixed venous blood. However, this increased O2 storage capacity would not affect the parameters of \({\dot{\text{V}}\text{O}}_{2}\), including \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), since the \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics during exercise of moderate intensity are not limited by oxygen delivery, but rather by intracellular (metabolic) factors. Subsequently, aerobically trained individuals are expected to have a higher O2 extraction fraction during supine cycling, compared to their untrained peers.
Methods
Participants
A total of 17 men volunteered to participate in the present study. The exclusion criteria were: arterial hypertension (≥ 140/90 mmHg), a history of cardiovascular or peripheral arterial disease, a history of neuromuscular injury, smoking, consumption of dietary supplements (creatine, whey protein and nitric oxide-based supplements) and/or medication use. Three participants were excluded based on these criteria leaving 14 healthy active men enrolled for data analysis. The study was approved by the Research Ethics Committee of the University of Split, Faculty of Kinesiology (Approval number: 2181205-02-05-20-020) and followed all the principles of the Declaration of Helsinki. Before signing the written consent, all participants were fully informed about the study procedures, protocols, and potential risks involved.
Study design
All experiments were conducted in the early afternoon hours in controlled laboratory settings. All participants arrived to the laboratory on three separate occasions with at least 48 h between sessions. The first visit encompassed preliminary medical screening that included taking medical history, and measuring resting blood pressure (BP), oxygen saturation (SpO2, Dash 2000, GE, Milwaukee, USA), and electrocardiography (ECG). After receiving medical clearance, participants completed a familiarization session on the cycle-ergometer to minimize learning effects, and avoid underestimation of \({\dot{\text{V}}\text{O}}_{2}\) uptake during the study. Participants were instructed to abstain from vigorous exercise, caffeine or alcohol consumption for 24 h prior to all experimental sessions. They also followed a standardized food intake to minimize variability in glucose oxidation as described elsewhere (Colosio et al. 2020). In the second session, a ramp incremental test (20 W min-1 increment) to voluntary exhaustion was performed on a cycle ergometer to determine \({\dot{\text{V}}\text{O}}_{2}\) max., power output (PO) attained at \({\dot{\text{V}}\text{O}}_{2}\) max., and heart rate (HR) max. In the final session, data were collected in triplicate from supine cycling in the step-transition test, starting at 20 W and reaching moderate-intensity PO, equivalent to \({\dot{\text{V}}\text{O}}_{2}\) at 90% GET.
We took special attention to standardize all supine cycling procedures on custom-designed setup and data collection protocols. The distances between the crankshaft, shoulder and pelvis distance were recorded for each participant, and then replicated throughout the study (Goulding et al. 2020a, b; Goulding et al. 2021; Zubac et al. 2021a). To reduce torso movement in the supine position and to improve ultrasonographic imaging of the spleen all participants were restrained to a bed during the supine position. The straps were positioned so that they did not interfere with breathing, but restricted excessive upper body movement and minimize separation from the saddle during the most strenuous pedaling toward the PPO and \({\dot{\text{V}}\text{O}}_{2}\) max. After determining \({\dot{\text{V}}\text{O}}_{2}\) max., all participants were assigned by the researchers to either an aerobically untrained (AU, n = 7) or trained (AT, n = 7) group, depending on the median value of their \({\dot{\text{V}}\text{O}}_{2}\) max. readings, as previously reported (Zubac et al. 2021b). The assignment to one of these groups was not known to the participants. All ultrasound examinations were performed by a trained cardiologist, with experience in abdominal ultrasonography, while in parallel, all participants carried out a step-transition cycling from 20 W (lasting 5 min.) to a moderate-intensity PO (90% GET, for 6 min.), that caused O2 uptake to increase to a level corresponding to 90% GET. This step-transition protocol was repeated three times, interspersed with 20 min. of rest, in agreement with previously published work (Zubac et al. 2021a). An overview of the data collection is shown in Fig. 1. During these step-transitions pulmonary \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) was measured breath-by-breath, while cardiovascular responses (via Finapres), and muscle oxygenation (via NIRS) were recorded simultaneously in Chart 5.0 software (ADInstruments, Castle Hill, Australia). In parallel, minute-by-minute ultrasonic measurements of the spleen were performed, at rest, during exercise and recovery in successive, 3 s. time frames. Blood samples were collected from the antecubital vein before and immediately after the third repetition of the step-transition protocol, processed and analyzed according to the previously published work (Bakovic et al. 2009).
Cardiopulmonary exercise testing
During preparation for CPET, participants rested quietly on the bed, while being instrumented with the metabolic analyzer mask (Hans Rudolph, Kansas City, USA) and the HR belt. The experimental protocol consisted of two successive, moderate-intensity step-transitions from 20 to 100 W, followed by a ramp incremental test until voluntary exhaustion, as recently published by Iannetta et al. (2019). The cycling cadence was set at 75 rpm. After completion of the incremental test, participants performed a 5-min cool down at reduced load (40 W). Pulmonary gas exchange was determined using the cardiopulmonary exercise testing unit (K-5, Cosmed, Rome, Italy), synchronized with the cycle-ergometer (Ergoline 900, Hamburg, Germany). Calibration of the metabolic unit was performed in agreement with the manufacturer's guidelines. All gas exchange/respiratory parameters were evaluated breath-by-breath, and HR was monitored with a Garmin device (HRM-3 SS, Kansas, USA).
Spleen imaging
The ultrasound imaging of the spleen was performed by the same cardiologist with experience in abdominal ultrasonography. Images were acquired with a GE M5S-D cardiac sector array probe (bandwidth 1.5–4.5 MHz, Vivid E9, GE HealthCare, USA) at baseline, during the first minute, at midpoint (3 min) and during last minute of 20 W cycling, and thereafter at each minute of the 90% GET supine cycling protocol, and during the recovery period at 1, 5, 10, 15 and 20 min. All data were stored for later analysis following previously published procedures (Palada et al. 2007; Bakovic et al. 2009, 2012). Three readings of spleen length and width boundaries were identified manually using an electronic caliper by the same author and pooled for further analysis. The intra-assay reliability of the spleen imaging during the study was satisfactory (CV = 2%). Splenic volumes from the images were calculated as originally suggested by Koga and Morikawa (1975), Koga (1979), and later adopted in our previous work (Palada et al. 2007; Bakovic et al. 2012; Zubac et al. 2021a).
Near infrared spectroscopy
During the on-transient cycling, time-course changes in local tissue oxygenation levels were recorded from the superficial portion of the VL by NIRS unit (NIRO-200, Hamamatsu Photonics KK, Tokyo, Japan). The NIRO-200 uses laser diodes set at four different wavelengths (776, 826, 845, and 905 nm) to provide the light source signal, which is pulsed in rapid succession and detected by the photomultiplier tube. The optical path length for the VL was taken from previously published work on NIRS signal acquisition during on-transient exercises (DeLorey et al. 2004; Zubac et al. 2021c). Optodes were positioned in a black rubber housing that ensured constant spacing of optodes on previously shaved skin. The housing was placed on the skin and fixed with micropore tape to minimize light artifacts. The pair of emitting and detecting optodes of the NIRS unit was placed over the right side of the participant's VL, while the participant was lying still on the bed. Parameters from NIRS (ΔHHb, ΔO2HHb) are expressed in micromoles as change from zero, while TOI (%) represents tissue oxygenation index.
Cardiovascular response
Continuous, non-invasive monitoring of the hemodynamics was performed by positioning Finometer cuff around the middle finger of the right hand (Finometer, Finapress Medical Systems, Arnhem, the Netherlands). The accuracy of BP monitoring was additionally calibrated against the mercury sphygmomanometer at the beginning of each session. The cuff of the photoplethysmograph was positioned and maintained at the level of the heart throughout the cycling protocol. The photoplethysmograph has been previously reported to accurately records changes in the MAP during exercise. From the continuous BP assessment, the arterial pulse wave was analyzed using a pulse wave analysis method that calculates changes in left ventricular stroke volume (SV) from the pulsatile systolic area. The improved method of Wesseling with the Modelflow program was used (model-based measurement method based on a nonlinear, 3-element model of the input impedance of the aorta) (Jellema et al. 1999). Cardiac output (CO) was computed as SV times HR, while the total peripheral resistance (TPR) was calculated as MAP divided by CO.
Blood collection protocol
Blood samples (∼ 6 mL) were collected by experienced personnel from the antecubital fossa of the participant’s left arm at baseline and immediately after completion of the exercise. Samples were collected in BD vacutainers containing dipotassium ethylenedinitrilotetraacetic acid, in agreement with previously published protocols (Bakovic et al. 2009; 2012). These samples were then analyzed within the next 2 h, using an automated hematology analyzer (ADVIA 2120i., Global Siemens Healthcare GmbH, Erlangen Germanya) in the Department of Medical Laboratory Diagnostics, University Hospital Center Split.
Data processing and analysis
Ramp incremental test: GET was determined independently by two exercise physiologists via V-slope method (Beaver et al. 1986). The influence of delayed \({\dot{\text{V}}\text{O}}_{2}\) was individually corrected for each participant (via mean response time, MRT), to calculate the power output associated with \({\dot{\text{V}}\text{O}}_{2}\) at GET, as originally proposed by Whipp and Wasserman (1972) and recently reported by Iannetta et al. (2019). The MRT calculation used avoids methodological shortcomings associated with determining GET during a single-visit CPET (Keir et al. 2015; Iannetta et al. 2019). Briefly, the GET was determined using the following criteria: a systematic increase in ventilatory equivalent of oxygen uptake (\({\dot{\text{V}}}_{{\text{E}}}\)/\({\dot{\text{V}}\text{O}}_{2}\)) and end-tidal pressure of oxygen without a concomitant increase in ventilatory equivalent of carbon dioxide production (\({\dot{\text{V}}}_{{\text{E}}}\)/\({\dot{\text{V}}\text{CO}}_{2}\)). The \({\dot{\text{V}}\text{O}}_{2}\) max. was determined via rolling average readings (20 s interval), recorded during the last minute of the incremental ramp test, whereas PPO and HR max. were defined as data attained at the end of the test(s).
Moderate-intensity step transitions: breath-by-breath \({\dot{\text{V}}\text{O}}_{2}\) data were visually inspected for outliers, (> 3 SDs from the local mean, based on six consecutive breaths) and removed if present. Next, raw data were aligned into 1-s bins, and then averaged every 5-s for each participant individually, with time-zero representing the onset of the on-transient exercise. The \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics data collected during the first 20 s of the step-transition protocol were excluded from the mono-exponential modeling, because the analysis of \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics in our study focused on the ‘fundamental’ component of the \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) rise (Mezzani et al. 2010). The \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) parameters at 90% GET (\(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), time delay, \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics amplitude) were modeled using mono-exponential, least-squares regression (Eq. 1), from the fundamental component onset to the 4th minute, when steady-state was reached, as suggested in the literature (DeLorey et al. 2004; Mezzani et al. 2010) and previously used by our laboratory (Zubac et al. 2021a, c). The equation parameters are explained in detail elsewhere (DeLorey et al. 2004; Mezzani et al. 2010; Zubac et al. 2021c). Briefly, Y(t) indicates \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) (or ΔHHb), at any given time-point; YBSLN is the baseline during cycling at 20 W (~ 60 s prior to step-transition), Amp is the steady-state rise in \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\); tau (τ) is the time constant marked as the time needed for \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) (or ΔHHb) to rise to 63% of the steady-state; and TD is the time delay. The muscle oxygenation data (presented via ΔHHb) were also time-aligned, and modeled in the same manner as the \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), except that the first 20 s were not removed from the analysis, whereas the ΔHHb fitting was modeled up to the 3rd minute of cycling, as previously described by DeLorey et al. (2004). Apart from the modeling procedures, data collected throughout the entire experimental protocol, including \({\dot{\text{V}}\text{O}}_{2}\), \({\dot{\text{V}}\text{CO}}_{2}\), \({\dot{\text{V}}}_{{\text{E}}}\), PO at 90% GET, TOI (%) and cardiovascular response were averaged across the last minute of each data collection phase including: baseline, 20 W warm-up, and cycling at 90% GET and recovery in agreement with previously published work (Zubac et al. 2021c). These data were then exported to an excel sheet and computed to provide an individualized response.
Statistics
All data were analyzed using Statistica version 13.0 (StatSoft, Tulsa, OK, USA) and presented as mean ± SD. Where appropriate, effect size (η2) was also reported, while normality of the distribution was confirmed using the Shapiro–Wilk test. Student’s independent t test was applied to detect differences between aerobically trained and untrained individuals across all dependent variables of interest. Homogeneity of variances was confirmed by Levene’s test. The effects of group (aerobically trained versus untrained) and time on various physiological parameters during on-transient cycling (e.g., spleen volume, cardiovascular response, and muscle oxygenation, \({\dot{\text{V}}\text{O}}_{2}\) utilization) were analyzed using a two-way ANOVA. If a significant F-test was identified, a Bonferroni post-hoc test was used to locate differences in time for each dependent variable was applied to determine multiple comparisons. Pearson’s linear correlations were calculated between spleen parameters (spleen volume at rest, and relative magnitude of spleen volume change) versus \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) and hematocrit values at rest.
Results
Fourteen, healthy young men completed all study procedures. No differences were found between groups in chronological age and stature, but the untrained individuals were ~ 7 kg heavier (p = 0.014), compared to their aerobically trained peers. No differences were found in resting cardiovascular parameters between groups. Mean resting values for HR and MAP were both within the normal range. The differences in cardiorespiratory response to the incremental ramp test between the aerobically trained and untrained individuals are given in Table 1. Briefly, \({\dot{\text{V}}\text{O}}_{2}\) max. (p = 0.021) was significantly lower in aerobically untrained, compared to trained individuals, even when corrected for body mass (by ~ 10 mL kg min−1, p = 0.001). The average \({\dot{\text{V}}\text{O}}_{2}\) max. was 41.4 ± 4.9 (95% CI 37.3–44.7) for untrained, and 51.4 ± 3.0 (95% CI 48.9–53.4) for aerobically trained individuals. The median value for \({\dot{\text{V}}\text{O}}_{2}\) max. was 47.7 mL kg min−1. In parallel, no differences were observed between the groups in \({\dot{\text{V}}}_{{\text{E}}}\), \({\dot{\text{V}}\text{CO}}_{2}\), RER, and HR max., despite significant differences in peak PO (3.8 W kg−1 vs. 3.0 W kg−1, p = 0.001). Metabolic, cardiorespiratory, and muscle oxygenation index data during on-transient supine cycling at 90% GET are given in Table 2. No differences were observed between the two groups in \({\dot{\text{V}}}_{{\text{E}}}\), \({\dot{\text{V}}\text{O}}_{2}\), \({\dot{\text{V}}\text{CO}}_{2}\), and PO, and all parameters of interest transiently increased in response to step-transition cycling, and remained elevated until the end of exercise. Peak O2 uptake observed during the on-transient cycling was similar between groups (~ 2.8 L min−1). A significant interaction effect was identified between group x time (p = 0.001, η2 = 0.340) in the TOI (%) readings. Post hoc analysis showed that aerobically trained individuals had 6–8% (p = 0.001) lower TOI (%), throughout the on-transient protocol at 90% GET (Table 2), compared to untrained individuals.
No differences were observed between the two groups for \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics parameters during moderate-intensity supine step-transitions, including mathematically modeled baseline, amplitude, time delay or τ-time-constant. However, the \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) time-constant was 9 s. slower in untrained compared to trained individuals (NS, p = 0.086). Time-course changes in ΔHHb showed differences between the aerobically trained and untrained individuals in the amplitude of VL deoxygenation, with the amplitude being ~ 30% (p = 0.001) greater in aerobically trained individuals. The time delay and τ-time-constant of the ΔHHb signal were similar between the groups, as presented in Table 3. The overall mono-exponential fitting (r2) was excellent, ranging from 0.94 to 0.99; for the \({\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics and from 0.97 to 0.99 for the ΔHHb parameters.
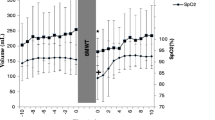
The magnitude of spleen volume reduction in response to supine, on-transient cycling was comparable between aerobically trained and untrained individuals (interaction effect: p = 0.641). More precisely, a transient reduction in spleen volume was observed in both groups, starting at the first minute of supine cycling (at 20 W), and reaching maximal reduction (by ~ 115 mL in aerobically trained, and by ~ 106 mL in untrained participants, with an overall reduction of ~ 38%, p = 0.001) in the 6th minute of supine cycling at 90% GET. The rate of spleen volume reduction was ~ 10–14 mL per minute of exercise during on-transient supine cycling. Spleen volume fully recovered after nearly 20 min of rest Fig. 2.
Following exercise cassation, mean hemoglobin, hematocrit, erythrocyte’s concentration and platelet count transiently increased by 3.11%, 3.89%, 3.90% and 5.31%, (p = 0.001, respectively) when compared to baseline. Overall, no differences in hematological parameters were observed between groups as shown in Fig. 3. To wit, a moderate increase in leukocytes (by ~ 24%, time effect: p = 0.002) was similar between the groups.
Data on cardiovascular response during the supine on-transient cycling are presented in Fig. 4. Briefly, no differences were observed between the aerobically trained and untrained individuals in the MAP, CO, and TPR. Only a transient change over time was observed in all reported variables, indicating immediate cardiovascular adjustments (~ 30 to 40% increase in MAP and CO, paralleled by a 35% decrease in TPR, time effect: p = 0.001) in response to the on-transient supine cycling.
In Fig. 5 no correlation was observed between spleen volume at rest versus \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) (panel A). Similarly, no correlation was observed for (i) greatest spleen volume reduction (%) versus \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) and (ii) spleen volume at rest versus oxygen uptake (presented in mL min−1, and averaged over the last two minutes) of supine cycling (Fig. 4, panels B, C). Also, the hematocrit (L/L) at baseline was not correlated with \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) (panel D).
Correlation analysis. Panel A—resting spleen volumes versus the oxygen uptake kinetics; Panel B—the magnitudes of spleen volume changes versus the time constant of the oxygen uptake kinetics; Panel C—the magnitudes of spleen volume changes versus the oxygen uptake at 90% of the gas exchange threshold; Panel D—baseline hematocrit versus the time constant of the oxygen uptake kinetics
In Fig. 6 a trace of pulmonary oxygen uptake kinetics (\(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\)) and ΔHHb kinetics during on-transient supine cycling for aerobically trained and untrained individual(s) are presented.
A representative trace of the pulmonary oxygen uptake kinetics (panels A and B, aerobically trained versus untrained individual) and NIRS-derived HHb signal (panels C and D, aerobically trained versus untrained individuals) during the on-transient supine cycling; \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) time constant of the oxygen uptake kinetics; NIRS near infrared spectroscopy: ΔHHb deoxygenated hemoglobin;
Discussion
We investigated the effects of a supine step-transition cycling at PO equivalent to 90% GET on different physiological parameters, including; spleen volume changes, O2 utilization, and hematological parameters in young healthy males of varying cardiorespiratory fitness. The present findings only partially support the study hypothesis, as no significant differences in the magnitude of spleen volume reduction were observed between aerobically trained and untrained individuals. A transient increase in circulating RBC concentration was observed, immediately after cycling-exercise cessation, with no significant differences between the studied groups. Correlation analysis showed that greater splenic emptying not correlate with does not argument faster pulmonary \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), regardless of subsequent RBC release. However, a ~ 30% greater deoxygenation amplitude (NIRS-derived ΔHHb kinetics) of the VL during the supine cycling at 90% GET was observed in aerobically trained compared to their untrained peers.
\(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) Importantly, the present work provides a novel insight into the magnitude of spleen volume reduction, as here we report for the first time in the literature the rate of the spleen volume reduction (10–14 mL per minute of exercise) during on-transient cycling exercise at 90% GET is reported.
This area has received less attention, because a clear image of the contracting spleen is sometimes difficult to obtain using ultrasound imaging. This is primarily due to the methodological drawbacks associated with splenic imaging, particularly during upright-posture exercise, as the anatomical position of the spleen implies isolated views in the region of interest, and likely overlaps with other organs. However, the present supine cycling-model evades different technical shortcomings, by allowing constant positioning of the probe in the region of interest. This allows each image to capture the spleen size adaptations associated with a given exercise intensity domain. Previously published work in this area, and especially those studies using ultrasound imaging to detect changes in splenic volume (Sprelich et al. 2015; Prommer et al. 2007), may have omitted some important information regarding the magnitudes of both splenic contractility and reactivity, and thereby underestimated the spleen emptying capacity (Sprelich et al. 2015), and even subsequent RBC release (Prommer et al. 2007). Here, no differences in spleen volume reduction were observed between groups, and this finding is to some extent inconsistent with previously published cross-sectional studies on spleen emptying during breath-hold diving (Bakovic et al. 2003; Prommer et al. 2007; Schagatay et al. 2020). Indeed, greater splenic emptying has been reported in the literature as an advantage of trained individuals (divers or trekkers, for example) over healthy active controls (between 5 and 30% difference in favor of trained individuals), likely due to their extensive training background and careful selection (Bakovic et al. 2003; Bouten et al. 2019; Schagatay et al. 2012). The differences between our and previous studies can be explained, at least in part, by the fact that consecutive apneas and supine aerobic exercise elicit different physiological responses. Briefly, to preserve O2 during apnea the diving reflex response is activated, characterized by vagally mediated bradycardia and sympathetic α-adrenergic peripheral vasoconstriction (Andersson et al. 2000; Palada et al. 2007). In contrast, moderate-intensity supine-cycling is associated with changes in muscle contractions (moderate muscle pump activation), rapid vasodilation, and redistribution of cardiac output to meet metabolic demands at the tissue level. Here, we observed no differences between the groups in their cardiovascular response to supine cycling exercise at PO equivalent to 90% GET, whereas cycling exercise was associated with a sudden increase in the readings of MAP and CO, respectively (Fig. 4). Another plausible explanation was recently presented by Illardo et al. (2018) in her work on the genetic adaptations of diving Bajau-Sea nomads from Southeast Asia. Using a comparative genomic study, her work showed that natural selection and genetic variants in the PDE10A gene contribute to a larger spleen in the Bajau. This allows them to mobilize a larger amount of O2-carrying RBC, and prolong the diving duration in the open sea. However, the role of the spleen in O2 transport during aerobic exercise has not been carefully studied. Moreover, direct evidence that splenic contraction also augments aerobic performance during exercise in humans is lacking, as there is still an on-going debate in the literature regarding the role of the spleen in O2 transport under different scenarios (Gatterer and Burtscher 2021; Schagatay and Holmström 2021; Holmström et al. 2021b). Therefore, the correlation between the spleen volume changes, circulating RBC release and \({\dot{\text{V}}\text{O}}_{2}\) uptake parameters was explored here. We found that greater splenic emptying does not argument faster pulmonary \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\), irrespective of the magnitude of the spleen volume reduction(s) and subsequent RBC release. Indeed, no associations existed between the (i) spleen volumes at rest (ii) spleen volume changes (%), O2 uptake parameters, and hematocrit at baseline (Fig. 5). In our brief report, a positive correlation (r = 0.759, p = 0.011) was observed between resting hematocrit and the \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics (Zubac et al. 2021c), whereas the data presented here contradict this finding. Thus, further investigation is required, although some authors believe that a positive correlation between baseline hematocrit and \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) kinetics supports the compelling weight of evidence that O2 delivery is not a constraint of \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) in young healthy individuals (Poole et al. 2007). Furthermore, data presented here are consistent with previous work examining blood/plasma transfusion and subsequent arterial blood O2-carrying capacity enhancement on parameters of \({\dot{\text{V}}\text{O}}_{2}\) kinetics (Burnley et al. 2006; Wilkerson et al. 2005; Gordon et al. 2010). In short, these papers provide strong evidence that manipulations of oxygen delivery through blood-related variables have no effect on \({\dot{\text{V}}\text{O}}_{2}\) kinetics during moderate, heavy, or severe intensity cycling exercise. Apparently, the expected increase of the O2 storage capacity in venous blood, available at the onset of the on-trainset supine cycling, was quite small (Fig. 3). Supportive data were also presented by Prommer and co-authors (2007), who indicated that a transient, 3%–5% increase in circulating RBC ejected from the spleen, does not increase the O2 storage in the blood. Overall, transient increase in the circulating Hb was rather insignificant (the average increase was ~ 5 g/L) and, as such, cannot be expected to affect substantially the \({\dot{\text{V}}\text{O}}_{2}\) utilization, especially since the \({\dot{\text{V}}\text{O}}_{2}\) uptake during exercise of moderate intensity is not limited by the oxygen delivery. Nonetheless, there is still some controversy, including the duration of activity and the time-frame from cessation of apnea/exercise to blood sampling, and this warrants further investigation. Even so, the data presented here provide robust evidence that splenic emptying, and subsequent erythrocyte release do not correlate with \(\tau {\dot{\text{V}}\text{O}}_{{2{\text{p}}}}\) or steady-state \({\dot{\text{V}}\text{O}}_{2}\) utilization in healthy young men during moderate-intensity supine cycling exercise (Fig. 5). In fact, our data contradict the recent series of letters to the editor by Schagatay and Holmström, (2021) and Engan et al. (2021), who suggested that splenic emptying augments O2 transport to active tissues (via RBC release), under all circumstances, including aerobic exercise. Our work also addresses, at least in part the on-going debate in the literature (Schagatay and Holmström, 2021; Gatterer and Burtscher 2021), regarding the role of the spleen in O2 transport under different scenarios, including standardized laboratory conditions at sea level.
The time-course changes in the VL deoxygenation (as determined from ΔHHb kinetics) were statistically different between groups, and the deoxygenation amplitude was 30% (p = 0.001) greater in trained compared to untrained individuals (Table 3) during supine on-transient cycling at 90% GET. Apparently, the enhanced vasodilation (e.g., enhanced diffusive O2 delivery), and greater microvascular O2 extraction in the VL muscle region, as observed by NIRS-derived variables (including TOI %), suggests that aerobically trained individuals are capable of superior microvascular O2 extraction, compared to their aerobically untrained counterparts (Poole 2007; Davis and Barstow 2013). The most plausible explanation is that the superficial portion of the VL muscle of aerobically trained young men is characterized with dense capillary networks, and a greater O2 diffusion capacity between capillary and myocytes (presumably due to the increased capillary exchange surface area, and greater capillary hematocrit present at the fiber surface), compared to untrained individuals (Poole 2007). Thus, it seems that participants with higher O2 diffusion capacity achieved higher \({\dot{\text{V}}\text{O}}_{2}\) max. during CPET, compared to less trained individuals (Table 1), and in particular during supine cycling-exercise where predominantly type II fibers are activated (Goulding et al. 2020b, 2021). These results also suggest that the increase in blood O2-carrying capacity via splenic emptying, and thus the potential for muscle O2 supply from a greater amount of circulating RBC’s has no effect on O2 kinetics during supine cycling at moderate intensity, inferring that the latter is mainly regulated by intracellular (metabolic) factors (Poole and Jones 2011; Grassi 2015), as recently demonstrated in series of papers by (Goulding et al. 2020b, 2021), at least in young healthy men.
The current study provides novel insight into acute splenic volume changes and its correlation with \({\dot{\text{V}}\text{O}}_{2}\) utilization during supine cycling. Nevertheless, the present results may be limited by several factors. The most important limitation is that we were unable to recruit more than fourteen healthy young males, primarily due to the outbreak of the COVID-19 epidemics. Next, we failed to recruit female participants, due to concerns of a high drop-out rate, due to COVID-19 infection or lockdowns. Studies involving female participants should be controlled for a specific cycle phase, to exclude any kind of bias in the data due to menstrual and hormonal influences, as hormonal fluctuations largely modulate the physiological response to exercise (Sims and Heather 2018). This is particularly noteworthy as the sympathetic innervation accounts for 98% of the nerve fibers innervating the spleen via the splenic nerve (Klein et al. 1982). Therefore, a strong recommendation for future studies is to investigate the sex- differences in various physiological parameters, including; spleen volume changes, supine kinetics of O2 uptake, NIRS responses, and hematological parameters. In addition, future studies should attempt to overcome the technical drawbacks associated with ultrasonic imaging of the spleen during exercise at heavy and severe intensity domains. These methodological challenges include caudal movements during deep inspiration, changes in intra-abdominal pressure and overlapping of the spleen with the diaphragm, and rib cage, likely due to splenic excursions within the abdominal cavity during vigorous cycling bouts. Overall, these factors preclude adequate visualization of the spleen, and in particular spleen thickness, which is often measured by others (Schagatay E et al. 2020; Holmström et al. 2021). However, this may further complicate attempts to capture spleen size via a single image during aerobic exercise.
Conclusion
In conclusion, no significant differences in the magnitude of spleen volume reduction were observed between aerobically trained and untrained participants. Furthermore, this was the first study to quantify the rate of splenic volume reduction (10–14 mL per min) during supine step-transition cycling at a given moderate-intensity domain. Also, the present results suggest that the exercise-induced splenic emptying, does not play an important role in O2 transport in healthy young man of varying cardiorespiratory fitness. Thus, despite a popular concept in the current literature, splenic emptying does not correlate with O2 utilization parameters during moderate-intensity aerobic exercise under standardized laboratory conditions at sea level. This is further supported by the fact that aerobically trained individuals are capable of greater O2 extraction at the level of VL (as reflected by NIRS-derived variables), compared to their aerobically untrained peers. Overall, our work addresses the on-going debate in the literature, by demonstrating that O2 utilization during supine on-transient exercise at moderate-intensity is not enhanced via larger splenic emptying, and subsequent RBC release. Rather, O2 utilization during supine cycling in healthy young men is limited primarily by skeletal muscle bioenergetics.
Change history
10 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00421-022-04893-8
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- CO:
-
Cardiac output
- CPET:
-
Cardiopulmonary exercise testing
- DBP:
-
Diastolic blood pressure
- ECG:
-
Electrocardiography
- GET:
-
Gas exchange threshold
- HR:
-
Heart rate
- Hb:
-
Hemoglobin
- Hct:
-
Hematocrit
- HHb:
-
Deoxygenized hemoglobin
- MRT:
-
Mean response time
- MAP:
-
Mean arterial pressure
- NIRS:
-
Near infrared spectroscopy
- O2 :
-
Oxygen
- PO:
-
Power output
- PPO:
-
Power output attained at \({\dot{\text{V}}\text{O}}_{2}\) max.
- RBC:
-
Red blood cells
- RER:
-
Respiratory exchange ratio
- SBP:
-
Systolic blood pressure
- SV:
-
Stroke volume
- TOI:
-
Total oxygenation index
- TPR:
-
Total peripheral resistance
- \({\dot{\text{V}}}_{{\text{E}}}\) :
-
Peak pulmonary ventilation
- \({\dot{\text{V}}\text{O}}_{2}\) :
-
Oxygen utilization
- \({\dot{\text{V}}\text{O}}_{2}\) max.:
-
Maximal oxygen uptake
- \({\dot{\text{V}}\text{CO}}_{2}\) :
-
Carbon dioxide production
- VL:
-
Vastus lateralis
References
Allsop P, Peters AM, Arnot RN, Stuttle AWJ, Deenmamode M, Gwilliam ME, Hall GM (1992) Intrasplenic blood cell kinetics in man before and after brief maximal exercise. Clin Sci 83(1):47–54
Andersson J, Schagatay E, Gislen A, Holm B (2000) Cardiovascular responses to cold water immersions of the forearm and face, and their relationship to apnoea. Eur J Appl Physiol 83:566–572
Bakovic D, Valic Z, Eterovic D, Vukovic I, Obad A, Marinovic-Terzic I, Dujic Z (2003) Spleen volume and blood flow response to repeated breath-hold apneas. J Appl Physiol 95(4):1460–1466. https://doi.org/10.1152/japplphysiol.00221.2003
Bakovic D, Pivac N, Eterovic D, Palada I, Valic Z, Paukovic-Sekulic B, Dujic Z (2009) Changes in platelet size and spleen volume in response to selective and non-selective Β-adrenoceptor blockade in hypertensive patients. Clin Exp Pharmacol Physiol 36(4):441–446. https://doi.org/10.1111/j.1440-1681.2008.05090.x
Bakovic D, Pivac N, Eterovic D, Breskovic T, Zubin P, Obad A, Dujic Z (2012) The effects of low-dose epinephrine infusion on spleen size, central and hepatic circulation and circulating platelets. Clin Physiol Funct Imaging 33(1):30–37. https://doi.org/10.1111/j.1475-097X.2012.01156.x
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60(6):2020–2027. https://doi.org/10.1152/jappl.1986.60.6.2020
Bouten J, Caen K, Stautemas J, Lefevere F, Derave W, Lootens L, Van Aenoo P, Bourgois JG, Boone J (2019) Eight weeks of static apnea training increases spleen volume but not acute spleen contraction. Respir Physiol Neurobiol 266:144–149. https://doi.org/10.1016/j.resp.2019.04.002
Burnley M, Roberts CL, Thatcher R, Doust JH, Jones AM (2006) Influence of blood donation on O2 uptake on-kinetics, peak O2 uptake and time to exhaustion during severe-intensity cycle exercise in humans. Exp Physiol 91(3):499–509
Colosio AL, Caen K, Bourgois JG, Boone J, Pogliaghi S (2020) Bioenergetics of the VO2 slow component between exercise intensity domains. Pflugers Arch Eur J Physol 472(10):1447–1456
Davis ML, Barstow TJ (2013) Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. Respir Physiol Neurobiol 186(2):180–187
DeLorey DS, Kowalchuk JM, Paterson DH (2004) Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol 97:165–172. https://doi.org/10.1152/japplphysiol.01179.2003
Elia A, Barlow MJ, Wilson OJ, O’Hara JP (2021) Splenic responses to a series of repeated maximal static and dynamic apnoeas with whole-body immersion in water. Exp Physiol 106(1):338–349
Engan H, Patrician A, Schagatay E (2021) Letter to the editor: on the increased haemoglobin concentration and improved oxygen uptake after Spirulina supplementation. Eur J Appl Physiol. https://doi.org/10.1007/s00421-021-04649-w
Espersen K, Frandsen H, Lorentzen T, Kanstrup IL, Christensen NJ (2002) The human spleen as an erythrocyte reservoir in diving-related interventions. J Appl Physiol 92(5):2071–2079. https://doi.org/10.1152/japplphysiol.00055.2001
Gatterer H, Burtscher M (2021) Is splenic contraction more pronounced when exercising in hypoxia than normoxia? Eur J Appl Physiol. https://doi.org/10.1007/s00421-021-04703-7
Gordon D, Marshall K, Connell A, Barnes RJ (2010) Influence of blood donation on oxygen uptake kinetics during moderate and heavy intensity cycle exercise. Int J Sports Med 31(05):298–303
Goulding RP, Okushima D, Marwood S, Poole DC, Barstow TJ, Lei TH, Kondo N, Koga S (2020a) Impact of supine exercise on muscle deoxygenation kinetics heterogeneity: mechanistic insights into slow pulmonary oxygen uptake dynamics. J Appl Physiol 129(3):535–546. https://doi.org/10.1152/japplphysiol.00213.2020
Goulding RP, Okushima D, Marwood S, Poole DC, Barstow TJ, Lei TH, Koga S (2020b) Impact of supine exercise on muscle deoxygenation kinetics heterogeneity: mechanistic insights into slow pulmonary oxygen uptake dynamics. J Appl Physiol 129(3):535–546
Goulding RP, Okushima D, Fukuoka Y, Marwood S, Kondo N, Poole DC, Koga S (2021) Impact of supine versus upright exercise on muscle deoxygenation heterogeneity during ramp incremental cycling is site specific. Eur J Appl Physiol 121(5):1283–1296. https://doi.org/10.1007/s00421-021-04607-6
Grassi B (2005) Delayed metabolic activation of oxidative phosphorylation in skeletal muscle at exercise onset. Med Sci Sports Exerc 37(9):1567–1573
Holmström PK, Bird JD, Thrall SF, Kalker A, Herrington BA, Soriano JE, Day TA (2021a) The effects of high altitude ascent on splenic contraction and the diving response during voluntary apnoea. Exp Physiol 106(1):160–174. https://doi.org/10.1113/EP088571
Holmström PK, Karlsson Ö, Lindblom H, McGawley K, Schagatay EK (2021b) Enhanced splenic volume and contraction in elite endurance athletes. J Appl Physiol 131(2):474–486. https://doi.org/10.1152/japplphysiol.01066.2020
Hurford WE, Hong SK, Park YS, Ahn DW, Shiraki K, Mohri M, Zapol WM (1990) Splenic contraction during breath-hold diving in the Korean ama. J Appl Physiol 69(3):932–936
Iannetta D, Murias JM, Keir DA (2019) A simple method to quantify the VO2 mean response time of ramp-incremental exercise. Med Sci Sports Exerc 51(5):1080–1086. https://doi.org/10.1249/MSS.0000000000001880
Ilardo MA, Moltke I, Korneliussen TS, Cheng J, Stern AJ, Racimo F, Willerslev E (2018) Physiological and genetic adaptations to diving in sea nomads. Cell 173(3):569–580
Jellema WT, Wesseling KH, Groeneveld AB, Stoutenbeek CP, Thijs LG, van Lieshout JJ (1999) Continuous cardiac output in septic shock by simulating a model of the aortic input impedance: a comparison with bolus injection thermodilution. Anesthesiology 90:1317–1328. https://doi.org/10.1097/00000542-199905000-00016
Keir DA, Fontana FY, Robertson TC, Murias JM, Paterson DH, Kowalchuk JM, Pogliaghi S (2015) Exercise intensity thresholds: identifying the boundaries of sustainable performance. Med Sci Sports Exerc 47(9):1932–1940. https://doi.org/10.1249/MSS.0000000000000613
Klein B, Smadja-Joffe F, Pragnell I, Ostertag W, Jasmin C (1982) A study of added GM-CSF independent granulocyte and macrophage precursors in mouse spleen infected with myeloproliferative sarcoma virus (MPSV). Exp Hematol 10(4):373–382
Koga T (1979) Correlation between sectional area of the spleen by ultrasonic tomography and actual volume of the removed spleen. J Clin Ultrasound 7(2):119–120. https://doi.org/10.1002/jcu.1870070208
Koga T, Morikawa Y (1975) Ultrasonographic determination of the splenic size and its clinical usefulness in various liver diseases. Radiology 115(1):157–161. https://doi.org/10.1148/115.1.157
Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ (1999) Kinetics of oxygen uptake during supine and upright heavy exercise. J Appl Physiol 87:253–260
Lodin-Sundström A, Holmström P, Ekstam M, Söderberg D, Schagatay E (2021) Splenic contraction is enhanced by exercise at simulated high altitude. Eur J Appl Physiol. https://doi.org/10.1007/s00421-021-04637-0
Matsuda S, Uchida T (1989) Blood cell kinetics in the spleen. Rinsho Ketsueki 30:1261–1265
Mezzani A, Grassi B, Giordano A, Corrà U, Colombo S, Giannuzzi P (2010) Age-related prolongation of phase I of VO2 on-kinetics in healthy humans. Am J Physiol Regul Integr Comp Physiol 299(3):968–976. https://doi.org/10.1152/ajpregu.00739.2009
Murias JM, Kowalchuk JM, Paterson DH (2011) Speeding of VO2 kinetics in response to endurance-training in older and young women. Eur J Appl Physiol 111(2):235–243
Palada I, Eterović D, Obad A, Bakovic D, Valic Z, Ivancev V, Lojpur M, Shoemaker JK, Dujic Z (2007) Spleen and cardiovascular function during short apneas in divers. J Appl Physiol 103(6):1958–1963. https://doi.org/10.1152/japplphysiol.00182.2007
Poole DC, Jones AM (2011) Oxygen uptake kinetics. Compr Physiol 2(2):933–996. https://doi.org/10.1002/cphy.c100072
Poole DC, Ferreira LF, Behnke BJ, Barstow TJ, Jones AM (2007) The final frontier: oxygen flux into muscle at exercise onset. Exerc Sport Sci Rev 35(4):166–173
Prommer N, Ehrmann U, Schmidt W, Steinacker JM, Radermacher P, Muth CM (2007) Total haemoglobin mass and spleen contraction: a study on competitive apnea divers, non-diving athletes and untrained control subjects. Eur J Appl Physiol 101(6):753–759
Richardson RS, Wary C, Wray DW, Hoff J, Rossiter H, Layec G, Carlier PG (2015) MRS evidence of adequate O2 supply in human skeletal muscle at the onset of exercise. Med Sci Sports Exerc 47(11):2299–2307
Rossiter HB (2011) Exercise: kinetic considerations for gas exchange. Compr Physiol 1(1):203–244. https://doi.org/10.1002/cphy.c090010
Schagatay E, Holmström P (2021) Response to letter to editor by Gatterer and Burtscher concerning splenic contraction in different situations. Eur J Appl Physiol. https://doi.org/10.1007/s00421-021-04704-6
Schagatay E, Richardson MX, Lodin-Sundström A (2012) Size matters: spleen and lung volumes predict performance in human apneic divers. Front Physiol 3:173. https://doi.org/10.3389/fphys.2012.00173
Schagatay E, Holmström P, Mulder E, Limbu P, Schagatay FS, Engan H, Lodin-Sundström A (2020) Spleen volume and contraction during apnea in Mt. Everest climbers and Everest base camp trekkers. High Alt Med Biol 21(1):84–91
Sims ST, Heather AK (2018) Myths and methodologies: reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol 103(10):1309–1317. https://doi.org/10.1113/EP086797
Sperlich B, Zinner C, Pfister R, Holmberg HC, Michels G (2015) Repeated apnea-induced contraction of the spleen in cyclists does not enhance performance in a subsequent time-trial. Eur J Appl Physiol 115(1):205–212. https://doi.org/10.1007/s00421-014-3003-x
Stewart IB, Warburton DE, Hodges AN, Lyster DM, McKenzie DC (2003) Cardiovascular and splenic responses to exercise in humans. J Appl Physiol 94(4):1619–1626
Whipp BJ, Wasserman K (1972) Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol 33(3):351–356. https://doi.org/10.1152/jappl.1972.33.3.351
Wilkerson DP, Rittweger J, Berger NJ, Naish PF, Jones AM (2005) Influence of recombinant human erythropoietin treatment on pulmonary O2 uptake kinetics during exercise in humans. J Physiol 568(2):639–652. https://doi.org/10.1113/jphysiol.2005.089920
Zubac D, Obad A, Bosnjak A, Zec M, Ivancev V, Valic Z (2021a) Spleen emptying does not correlate with faster oxygen kinetics during a step-transition supine cycling. Appl Physiol Nutr Metab. https://doi.org/10.1139/apnm-2021-0294.2021
Zubac D, Obad A, Ivančev V, Valić Z (2021b) Acute flywheel exercise does not impair the brachial artery vasodilation in healthy men of varying aerobic fitness. Blood Press Monit 26(3):215–223. https://doi.org/10.1097/MBP.0000000000000523
Zubac D, Ivančev V, Valić Z, Šimunič B (2021c) Long-lasting exercise involvement protects against decline in V̇O2 max and V̇O2 kinetics in moderately active women. Appl Physiol Nutr Metab 46(2):108–116. https://doi.org/10.1139/apnm-2020-0307
Acknowledgements
The present work did not receive any specific grant from funding agencies in the public, commercial, or any other sector.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DZ, VI, ZV, MZ, AB, DŠD, and AO. The first draft of the manuscript was written by DZ and ZV, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Communicated by Guido Ferretti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: There are mistakes in the table 2.
Rights and permissions
About this article
Cite this article
Zubac, D., Obad, A., Šupe-Domić, D. et al. No differences in splenic emptying during on-transient supine cycling between aerobically trained and untrained participants. Eur J Appl Physiol 122, 903–917 (2022). https://doi.org/10.1007/s00421-021-04843-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04843-w