Abstract
Purpose
There is evidence of both the preventive effects and poor acceptance of mouthguards. There are various effects on performance depending on the type of mouthguard model. Hemodynamic responses to wearing a mouthguard have not been described. The aim of this study was to investigate the effects of self-adapted mouthguards with breathing channels (SAMGvent).
Methods
In this randomized crossover study, 17 healthy, active subjects (age 25.12 ± 2.19 years) underwent body plethysmography and performed two incremental exertion tests wearing a (SAMGvent) and not wearing (CON) a mouthguard. Blood lactate, spirometrics, and thoracic impedance were measured during these maximum exercise tests.
Results
The mean values using a SAMGvent revealed significantly greater airway resistance compared to CON (0.53 ± 0.16 kPa·L−1 vs. 0.35 ± 0.10 kPa·L−1, respectively; p = < 0.01). At maximum load, ventilation with SAMGvent was less than CON (118.4 ± 28.17 L min−1 vs. 128.2 ± 32.16 L min−1, respectively; p = < 0.01). At submaximal loads, blood lactate responses with SAMGvent were higher than CON (8.68 ± 2.20 mmol·L−1 vs. 7.89 ± 1.65 mmol·L−1, respectively; p < 0.01). Maximum performance with a SAMGvent was 265.9 ± 59.9 W, and without a mouthguard was 272.9 ± 60.8 W (p < 0.01). Maximum stroke volume was higher using a SAMGvent than without using a mouthguard (138.4 ± 29.9 mL vs. 130.2 ± 21.2 mL, respectively; p < 0.01).
Conclusion
Use of a self-adapted mouthguard led to increased metabolic effort and a significant reduction in ventilation parameters. Unchanged oxygen uptake may be the result of cardiopulmonary compensation and increased breathing efforts, which slightly affects performance. These results and the obvious preventive effects of mouthguards support their use in sports.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mouthguards (MGs) are a key factor in preventing sports-related dental injuries, especially in contact sports (Galic et al. 2018; Lässing et al. 2020b; Petrović et al. 2016). Various studies have demonstrated their preventive effect convincingly (ADA 2006; Bemelmanns and Pfeiffer 2000; Knapik et al. 2007; Lang et al. 2002; Mihalik et al. 2007). However, many athletes are very reluctant to wear mouthguards, largely because of both breathing restrictions (Amis et al. 2000; Bailey et al. 2015; Francis and Brasher 1991) and the fear of impairing performance (Caneppele et al. 2017; Delaney and Montgomery 2019). These limitations seem to depend on the model. There are two main types of mouthguards. Customized mouthguards are worn in professional sports and made individually by dentists. Inexpensive self-adapted mouthguards (SAMG) were designed for self-manufacture and widespread use, especially in youth sports (Kececi et al. 2005; Newsome et al. 2001). Some studies have postulated that using a customized mouthguard (CMG) exerts no negative effects on breathing (\(\dot{V}\)E), oxygen uptake (\(\dot{V}\)O2) or maximum performance compared to wearing a conventional self-adapted mouthguard, or not (Arent et al. 2010; Caneppele et al. 2017; Duarte-Pereira et al. 2008; El-ashke and El-ashker 2015; Morales et al. 2015). In activities involving and requiring high forces or metabolic energy efficiency, even the use of a CMG has demonstrated maximum ergogenic effects (Allen et al. 2018; Buscà et al. 2018; Garner and McDivitt 2009). Described are the hypothetical effects of CMG caused by an increase in airway diameter (Garner and McDivitt 2009) showing positive effects for gas exchange (Garner 2015; Garner et al. 2011; Schulze et al. 2019a), and enhancement through the jaw repositioning associated with beneficial effects on peripheral muscle innervation (Allen et al. 2018; Arent et al. 2010; Morales et al. 2015).
Regarding SAMG use, studies reveal some \(\dot{V}\)E restriction, but no negative effect on \(\dot{V}\)O2 or performances (Bailey et al. 2015; Francis and Brasher 1991; Schulze et al. 2019a,b,2020). In particular, the use of a specially designed SAMG with breathing channels (SAMGvent) led to—despite lower \(\dot{V}\)E—a lower blood lactate concentration (Bailey et al. 2015; Schulze et al. 2019a,b,2020). Yet other studies have confirmed negative effects on \(\dot{V}\)O2, \(\dot{V}\)E, and performance from using SAMGs compared to CMG (Bourdin et al. 2006; Caneppele et al. 2017; Duarte-Pereira et al. 2008; Lässing et al. 2020b; Arx et al. 2008).
Hemodynamic parameters associated with the use of mouthguards have not been measured to date. However, documenting these cardiac parameters might give us deeper insight into the effects of self–adapted mouthguard use—effects that might be closely associated with an increase in airway resistance (Bailey et al. 2015; Francis and Brasher 1991). The use of face masks also increases airway resistance, and has shown partially altered hemodynamic parameters (Fikenzer et al. 2020; Lässing et al. 2020a). The aim of this study was therefore to investigate the influence on hemodynamic and metabolic parameters of self-adapted mouthguards with breathing channels (SAMGvent). As the effects of wearing mouthguards on pulmonary parameters are known, we would expect a negative impact on performance.
Materials and methods
Ethical approval and study group
This study was reviewed and approved by the Ethics Committee of the Medical Faculty at the University of Leipzig (file number 445-15-21122015). All subjects with infectious, orthopedic, intrinsic or other diseases were excluded from this study.
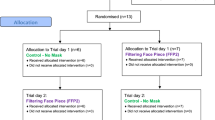
This prospective, randomized, crossover trial investigated the effects of a SAMGvent on cardiopulmonary, metabolic, and maximum power output in an ergometer step test compared to its execution without a mouthguard. The study included 17 healthy subjects (age 25.12 ± 1.9 years, weight 71.82 ± 10.50 kg and height 175.29 ± 8.04 cm). The group consisted of 8 men and 9 women who were sport students and who trained about 3.5 h a week. None of the subjects was a trained cyclist. Written informed consent was obtained from all participants. The subjects were advised not to train 24 h before the tests started, and to consume a specific amount of carbohydrates (men 10 g per kg BW and women 7 g per kg BW) to ensure that glycogen conditions remained stable.
Making of the mouthguards
The self-adapted mouthguard (Nike Adult Max Intake/Beaverton OR, USA) subjects wore is a non-customized mouthguard with breathing channels (SAMGvent). They were warmed up in boiling water (30 s) and pressed into the upper jaw by a specialist.
Body plethysmography
Body plethysmography (ZAN500 Body, nSpire Health GmbH, Germany) measurements were taken with the subject wearing a mask instead of a tube (Lässing et al. 2020c).
Pulmonary airway resistance (RAW) was tested randomly without a mouthguard and with the SAMGvent. Between these randomized tests, subjects were given a 5-min break so that their respiratory muscles could recover. The body plethysmography measurements were taken with the participants wearing multi-use silicone face masks with headgear (K4b2—face mask, Cosmed, Italy). The test person in Fig. 1 gave his written informed consent allowing his image to appear in an online publication.
Performance measures
The incremental exercise test was performed on two different days. We allowed an at least 2-day time interval between each test day.
Each test was started with 50 W for men and 30 W for women. Wattage was increased every minute by 15 W for men and 10 W for women up to the maximum possible load. All tests were performed on a semi-recumbent revolution independent cycle ergometer (ergometrics 900, Ergoline GmbH, Bitz, Germany) at 60–70 revolutions per minute. Cardiac output (CO), stroke volume (SV) and heart rate (HR) (measured by impedance cardiography; Physioflow, Manatec Biomedical, Macheren, France), maximum oxygen consumption (\(\dot{V}\)O2 max) and respiratory parameters (\(\dot{V}\)E, VT, RR) were monitored continuously at rest and during stress (K4b2, Cosmed, Italy). Spirometric and thoracic impedance data were averaged for 10 s over the load.
To monitor cardiac arrhythmias, the C5-lead ECG was continuously observed to ensure the subjects’ preventive forensic safety. Blood-lactate samples (20 µL) were taken every three minutes and subjected to enzymatic-amperometric measurement (Super GL, ISO 7550, Germany). Blood pressure (BP) was measured under rest, every three minutes under stress, and after the workload. Load intensity was classified as: “rest” (0 W), “moderate” (men = 215 W/women 170 W), “submaximal” (men MW = 320 W/women MW = 210 W) and individual “maximum”.
Calculations
Spirometric and thoracic impedance data were recorded as the 1-min average for each load level.
We calculated alveolar ventilation (\(\dot{V}\)A) by relying on the spirometrically recorded parameters that applied in these calculations (Bohr-formula): dead space volume (VD = VT × [FetCO2 (end-tidal fractional carbon dioxide concentration) − FeCO2 (mixed expired carbon dioxide concentration]/FetCO2), dead space ventilation (\(\dot{V}\)D = VD × RF); alveolar ventilation (\(\dot{V}\)A = (VT − VD) × RF). Breathing effort was calculated as follows: Intrapulmonary pressure = PEF × RAW. TPR was calculated: TPR = MAP/CO.
Statistical analysis
All values are presented as means with standard deviation. GraphPad Prism 8 (GraphPad Software Inc., California, USA) was used for statistical evaluations and graph preparation. The raw data from spirometry and impedance cardiography obtained continuously during exercise were synchronized and averaged over 10 s. The exercise parameters were then calculated for all subjects at moderate, submaximal and maximum load. For distribution analysis, the Kolmogorov–Smirnov normality test was used. If normality distribution was evident, statistical comparisons were made using paired parametric t test (body plethysmography, significance level was defined as p < 0.05) or repeated two-way ANOVA with Bonferroni´s post hoc test for multiple comparison (exercise parameter). Sphericity was determined-based on the epsilon value of the Geisser greenhouse (ε). If the sphericity was rejected, Greenhouse Geisser correction would apply.
Results
Body plethysmography measured with mask
Table 1 illustrates pulmonary parameters in the body plethysmography measurement with mask. Pulmonary function parameters showed no differences. Only airway resistance was significantly higher with the SAMG (Table 1).
Respiratory work was calculated relying on peak flow and airway resistance parameters, which revealed significant differences (CON 2.78 ± 0.8 kPa vs. SAMGvent 4.27 ± 1.7 kPa, p = < 0.01/ηp2 = 0.48).
Exercise testing
Baseline values were measured prior to each session (values not shown), and there were only TPR and Te significant differences in hemodynamics. 17 participants completed both tests. Figure 2 shows the time course of HR, SV, VE and Lac during the exercise tests with and without mouthgard. There were no significant differences in hemodynamics or metabolic parameters during moderate intensity. TPR was significantly lower at rest with SAMGvent (CON 15.62 ± 3.55 mmHg·L−1 vs. SAMGvent 14.15 ± 2.59 mmHg·L−1). Te was clearly prolonged under resting conditions with mouthguard use (CON 2.23 ± 1.13 s vs. SAMGvent 2.37 ± 1.12 s; p = 0.04). At submaximal intensity, LAC (CON 9.89 ± 1.65 mmol L−1 vs. SAMGvent 8.68 ± 2.20 mmol L−1; p < 0.01) and SV (CON 132.1 ± 20.9 mL vs. SAMGvent 139.4 ± 29.7 mL; p = 0.02) showed differences. All other measured parameters were at submaximal intensity not statistically different. Systolic and diastolic blood pressure revealed no differences throughout the exercise tests. Table 2 shows the maximum exercise parameters. The maximum power output achieved was lower with an SAMG. Pulmonary parameters differed significantly except for VO2 and VT. The SV was significantly increased and AVDO2 decreased when wearing an SAMGvent.
Discussion
Our study’s main finding was that wearing a self-adapted mouthguard significantly increases airway resistance (RAW) at rest and reduces the \(\dot{V}\)E during maximum load. Despite similar \(\dot{V}\)O2 values, we observed a small but significantly reduced maximum ergometer performance when the SAMGvent was worn. Cardiopulmonary and metabolic parameters (Fig. 2) may indicate primarily mechanical and less peripheral neural autonomic compensation to maintain \(\dot{V}\)O2 when wearing an SAMGvent.
Pulmonary parameter
Body plethysmography revealed that RAW rises significantly when wearing an SAMGvent. Other studies have also reported a significant or trending increase with MGs (Amis et al. 2000; Lässing et al. 2020c). Respiratory protection devices and breathing filters reveal similar effects (Lee and Wang 2011; Louhevaara 1984). Those studies demonstrate that increased RAW can also significantly reduce \(\dot{V}\)E during exercise, and lower the athlete’s performance (Fikenzer et al. 2020; Lässing et al. 2020c; Louhevaara 1984; Melissant et al. 1998). Such significantly lower \(\dot{V}\)E confirms the present study’s findings when using an SAMG (Bailey et al. 2015; Caneppele et al. 2017; Delaney and Montgomery 2019; Francis and Brasher 1991; Schulze et al. 2020). RF was also clearly reduced in conjunction with SAMGvent use, whereas VT was not influenced at maximum workload. Note that other studies have also reported lower RF with corresponding changes in breathing time when face-protection devices were used (Amis et al. 2000; Fikenzer et al. 2020; Francis and Brasher 1991; Lässing et al. 2020c; Louhevaara 1984; Schulze et al. 2020). Francis and Brasher (1991) suggest that a prolonged breathing cycle is a compensatory mechanism that can stabilize VT and the gas exchange when wearing an SAMG (Amis et al. 2000; Bailey et al. 2015; Lässing et al. 2020c). There is also evidence that CMG use had no effects on VT and \(\dot{V}\)O2 under maximum ergometer performance, but it did reduce \(\dot{V}\)E and extend Ti (Lässing et al. 2020c). According to Francis and Brasher (1991), a mechanism resembling the 'pursed lip' type of breathing (PLB) in patients with obstructed breathing lengthens the respiratory cycle time. The present results demonstrate reduced \(\dot{V}\)A, \(\dot{V}\)E, prolonged Ti, and lower performance with a SAMGvent compared to CON despite similar \(\dot{V}\)O2. The most likely explanation for these changes is the significantly increased airway resistance. Even more, the resulting greater breathing effort needed to maintain VE cancels some cardiopulmonal capacity, and might lead to distributional congruence between the respiratory and peripheral muscles (reduced AVDO2 and increased lactate) (Dominelli et al. 2017). The reduced ergometer performance despite unchanged VO2 may be attributable to this.
In the present study, the FetO2 was lower and FetCO2 clearly increased with the SAMGvent, compared to without a mouthguard. Some researchers have reported similar results, and assume an improved gas exchange rate when wearing a mouthguard (Garner et al. 2011; Schulze et al. 2020). Schulze et al. (2020) suspect that an altered jaw position favors innervation in the temporomandibular joint and associated dorsal muscle chain. They hypothesize that improved peripheral control stimulates the aerobic metabolic pathway, which may explain higher CO2 production per breath (Schulze et al. 2020). The present results indicate minor but significantly higher lactate levels, as well as 2.6% less maximum power output using an SAMGvent. The obstructive breathing patterns may be the reason for higher alveolar carbon dioxide partial pressure, represented by the FETCO2 value.
By wearing an SAMGvent higher RAW values lead to an altered exercise breathing pattern and significantly increased breathing capacity in healthy subjects, which limits \(\dot{V}\)A but not \(\dot{V}\)O2 (Bailey et al. 2015; Francis and Brasher 1991; Lässing et al. 2020c; Schulze et al. 2020).
Cardiocirculatory and metabolic parameters
There were no differences in HR parameters associated with wearing a mouthguard (Bailey et al. 2015; Delaney and Montgomery 2019; El-ashke and El-ashker 2015; Lässing et al. 2020c) in this study. Others have speculated that the PLB mechanism may influence performance when a mouthguard is worn (Amis et al. 2000; Bailey et al. 2015; Delaney and Montgomery 2019; Francis and Brasher 1991). We observed a higher SV in conjunction with SAMGvent use. Respiration is known to affect the SV (Convertino et al. 2005; Fikenzer et al. 2020; Jayaweera and Ehrlich 1987; Lässing et al. 2020c; Ryan et al. 2008). Some authors suspect that a longer Ti keeps pleural pressure on a negative level for longer, and may thus favor venous return (Jayaweera and Ehrlich 1987) during mouthguard use (Lässing et al. 2020c). Other studies have shown that increased inspiratory airway resistance can raise the SV (Convertino et al. 2005; Ryan et al. 2008). Increased respiratory muscle effort because of neural-reflex mechanisms could also be responsible for the rise in SV (Harms et al. 1998; Lee and Wang 2011). Unchanged blood pressure values and similar HRs suggest a more cardiopulmonary-mechanical than neural-reflex mechanism (Ryan et al. 2008). TPR’s mean values did not differ during exertion, thus supporting the assumption of a mechanical factor rather than a neuronal effect. As respiratory resistance induced a prolonged inspiratory phase, this could presumably increase the venous return flow and thus explain the mechanically-induced higher SV with enhancing effects on the \(\dot{V}\)O2 and maybe even the performance (Lässing et al. 2020c). The reduced AVDO2 during exercise is consistent with other studies reporting increased airway resistance when wearing face masks (Fikenzer et al. 2020; Lässing et al. 2020a). Reduced oxygen extraction caused by ventilatory obstruction has been suggested to be behind the increased lactate levels, and higher CO may due to afferent innervation from the working muscles (Blain et al. 2005; Busse et al. 1991; Harms et al. 1998). In contrast, independent studies demonstrated also the mechanical relationship between longer or higher negative pleural pressure and possible forcing effects on the transmural pressure difference in the extrathoracic and intrathoracic vessels (Convertino et al. 2005; Ryan et al. 2008) which may increase venous blood return and improve SV (Convertino et al. 2005; Fagoni et al. 2020; Fikenzer et al. 2020; Lässing et al. 2020a; Ryan et al. 2008).
In summary: the wearing of an SAMGvent led to an obstructed breathing pattern (Amis et al. 2000; Bailey et al. 2015; Francis and Brasher 1991; Lässing et al. 2020c) indicating slightly reduced maximum power (Caneppele et al. 2017; Duarte-Pereira et al. 2008; El-ashke and El-ashker 2015) without restricting \(\dot{V}\)O2 (Bailey et al. 2015; Francis and Brasher 1991; Kececi et al. 2005; Schulze et al. 2019a,2020). Mechanical cardiopulmonary compensation may contribute to stabilizing the \(\dot{V}\)O2 (Convertino et al. 2005; Lässing et al. 2020c; Ryan et al. 2008) which is probably higher because of the increased breathing effort while wearing a mouthguard than with no mouthguard. Nevertheless, the performance of participants wearing an SAMGvent in this study revealed moderate restrictions, probably because of the respiratory muscles’ higher oxygen consumption. As a similar study (Lässing et al. 2020c) employing customized mouthguards (CMG) reported no reduction in performance, we conclude that CMGs are preferable to the SAMGvent in this study.
Study limitations
The cardiac parameters we obtained via impedance cardiography may have been overestimated using absolute values (Siebenmann et al. 2015). However, since we compared intra-individual differences and impedance cardiography is so reliable (Astorino et al. 2015; Richard et al. 2001), changes in these parameters were essential, unlike those achieved using absolute values. Since to enable separate gender-specific data we would have needed a much larger cohort of study subjects, we cannot evaluate gender-specific differences. Nevertheless, our analyses show large homogeneity in the variation in variance of all means. Furthermore, our work does not take into account long-term adaptive regulations using a mouthguard, since the subjects wore the mouthguard only for these examinations.
Conclusion
Our investigation revealed increased airway resistance under resting conditions and significantly reduced respiratory parameters under stress in conjunction with wearing an SAMGvent. Maximum power output dropped slightly also, while the blood lactate concentration was higher. Oxygen uptake was unchanged and stroke volume improved, factors that potentially indicate cardiopulmonary compensation in combination with increased breathing effort. Nevertheless, we have demonstrated that wearing an SAMGvent reduces performance moderately—a factor that should be considered when these models are being used in sports.
Abbreviations
- ADA:
-
Access, prevention and interprofessional relations
- AVDO2 :
-
Arteriovenous oxygen difference
- CAP:
-
Concurrent activation potentiation
- CO:
-
Cardiac output
- FetCO2 :
-
End-tidal fractional carbon dioxide concentration
- FetO2 :
-
End-tidal fractional oxygen concentration
- FEV1 :
-
Forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- HR:
-
Heart rate
- SAMGvent :
-
Self-adapted mouthguard with breathing channels
- CON:
-
Control (without mouthguard)
- PEF :
-
Peak expiratory flow
- PIF :
-
Peak inspiratory flow
- RAW :
-
Airway resistance
- RF:
-
Respiratory frequency
- RQ:
-
Respiratory quotient
- SD:
-
Standard deviation
- SV:
-
Stroke volume
- T e :
-
Expiratory time
- T i :
-
Inspiratory time
- TPR:
-
Total peripheral resistance
- \(\dot{V}\) A :
-
Alveolar ventilation
- VC:
-
Vital capacity
- \(\dot{V}\)CO2 :
-
Carbon dioxide production
- \(\dot{V}\) E :
-
Ventilation
- \(\dot{V}\)O2 :
-
Oxygen uptake
- \(\dot{V}\)O2max :
-
Maximum oxygen uptake
- V T :
-
Tidal volume
- W:
-
Watt
References
ADA Council on Access, Prevention and Interprofessional Relations, ADA Council on Scientific Affairs (1939) Using mouthguards to reduce the incidence and severity of sports-related oral injuries. J Am Dent Assoc 1939 137:1712–1720. https://doi.org/10.14219/jada.archive.2006.0118(quiz 1731)
Allen CR, Fu Y-C, Cazas-Moreno V, Valliant MW, Gdovin JR, Williams CC, Garner JC (2018) Effects of jaw clenching and jaw alignment mouthpiece use on force production during vertical jump and isometric clean pull. J Strength Cond Res 32:5–11. https://doi.org/10.1519/JSC.0000000000002172
Amis T, Di Somma E, Bacha F, Wheatley J (2000) Influence of intra-oral maxillary sports mouthguards on the airflow dynamics of oral breathing. Med Sci Sports Exerc 32:284–290
Arent SM, McKenna J, Golem DL (2010) Effects of a neuromuscular dentistry-designed mouthguard on muscular endurance and anaerobic power. Comp Exerc Physiol 7:73–79. https://doi.org/10.1017/S1755254010000231
Astorino TA, Bovee C, DeBoe A (2015) Estimating hemodynamic responses to the wingate test using thoracic impedance. J Sports Sci Med 14:834–840
Bailey SP, Willauer TJ, Balilionis G, Wilson LE, Salley JT, Bailey EK, Strickland TL (2015) Effects of an over-the-counter vented mouthguard on cardiorespiratory responses to exercise and physical agility. J Strength Cond Res 29:678–684. https://doi.org/10.1519/JSC.0000000000000668
Bemelmanns P, Pfeiffer P (2000) Incidence of dental, mouth, and jaw injuries and the efficacy of mouthguards in top ranking athletes. Sportverletz Sportschaden Organ Ges Orthopadisch-Traumatol Sportmed 14:139–143. https://doi.org/10.1055/s-2000-8950
Blain G, Meste O, Bermon S (2005) Influences of breathing patterns on respiratory sinus arrhythmia in humans during exercise. Am J Physiol Heart Circ Physiol 288:H887-895. https://doi.org/10.1152/ajpheart.00767.2004
Bourdin M, Brunet-Patru I, Hager P-E, Allard Y, Hager J-P, Lacour J-R, Moyen B (2006) Influence of maxillary mouthguards on physiological parameters. Med Sci Sports Exerc 38:1500–1504. https://doi.org/10.1249/01.mss.0000228952.44850.eb
Buscà B, Moreno-Doutres D, Peña J, Morales J, Solana-Tramunt M, Aguilera-Castells J (2018) Effects of jaw clenching wearing customized mouthguards on agility, power and vertical jump in male high-standard basketball players. J Exerc Sci Fit 16(1):5–11. https://doi.org/10.1016/j.jesf.2017.11.001
Busse MW, Maassen N, Konrad H (1991) Relation between plasma K+ and ventilation during incremental exercise after glycogen depletion and repletion in man. J Physiol 443:469–476
Caneppele T, Borges A, Pereira D, Fagundes A, Fidalgo T, Maia L (2017) Mouthguard use and cardiopulmonary capacity—a systematic review and meta-analysis. Sports Med Int Open 1:E172–E182. https://doi.org/10.1055/s-0043-117599
Convertino VA, Cooke WH, Lurie KG (2005) Inspiratory resistance as a potential treatment for orthostatic intolerance and hemorrhagic shock. Aviat Space Environ Med 76:319–325
Delaney JS, Montgomery DL (2019) (5) Effect of noncustom bimolar mouthguards on peak ventilation in ice hockey players [Online]. ResearchGate: [date unknown]. https://www.researchgate.net/publication/7869932_Effect_of_Noncustom_Bimolar_Mouthguards_on_Peak_Ventilation_in_Ice_Hockey_Players. Accessed 2 Feb 2019
Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat-Seon Y, Henderson WR, Koehle MS, Boushel R, Sheel AW (2017) Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102:1535–1547. https://doi.org/10.1113/EP086566
Duarte-Pereira DMV, Del Rey-Santamaria M, Javierre-Garcés C, Barbany-Cairó J, Paredes-Garcia J, Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C (2008) Wearability and physiological effects of custom-fitted vs self-adapted mouthguards. Dent Traumatol Off Publ Int Assoc Dent Traumatol 24:439–442. https://doi.org/10.1111/j.1600-9657.2008.00595.x
El-ashke A, El-ashker S (2015) (9) Effect of mouthguard use on metabolic and cardiorespiratory responses to aerobic exercise in males [Online]. ResearchGate. https://www.researchgate.net/publication/324764411_Effect_of_Mouthguard_Use_on_Metabolic_and_Cardiorespiratory_Responses_to_Aerobic_Exercise_in_Males. Accessed 22 Nov 2018
Fagoni N, Bruseghini P, Adami A, Capelli C, Lador F, Moia C, Tam E, Bringard A, Ferretti G (2020) Effect of lower body negative pressure on phase I cardiovascular responses at exercise onset. Int J Sports Med 41:209–218. https://doi.org/10.1055/a-1028-7496
Fikenzer S, Uhe T, Lavall D, Rudolph U, Falz R, Busse M, Hepp P, Laufs U (2020) Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin Res Cardiol 109(12):1522–1530
Francis KT, Brasher J (1991) Physiological effects of wearing mouthguards. Br J Sports Med 25:227–231
Galic T, Kuncic D, Poklepovic Pericic T, Galic I, Mihanovic F, Bozic J, Herceg M (2018) Knowledge and attitudes about sports-related dental injuries and mouthguard use in young athletes in four different contact sports-water polo, karate, taekwondo and handball. Dent Traumatol Off Publ Int Assoc Dent Traumatol 34:175–181. https://doi.org/10.1111/edt.12394
Garner DP (2015) Effects of various mouthpieces on respiratory physiology during steady-state exercise in college-aged subjects. Gen Dent 63:30–34
Garner DP, McDivitt E (2009) Effects of mouthpiece use on airway openings and lactate levels in healthy college males. Compend Contin Educ Dent Jamesburg NJ 1995 30(Spec No 2):9–13
Garner DP, Dudgeon WD, Scheett TP, McDivitt EJ (2011) The effects of mouthpiece use on gas exchange parameters during steady-state exercise in college-aged men and women. J Am Dent Assoc 1939(142):1041–1047. https://doi.org/10.14219/jada.archive.2011.0325
Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA (1998) Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol Bethesda Md 1985 85:609–618. https://doi.org/10.1152/jappl.1998.85.2.609
Jayaweera AR, Ehrlich W (1987) Changes of phasic pleural pressure in awake dogs during exercise: potential effects on cardiac output. Ann Biomed Eng 15:311–318. https://doi.org/10.1007/bf02584286
Kececi AD, Cetin C, Eroglu E, Baydar ML (2005) Do custom-made mouth guards have negative effects on aerobic performance capacity of athletes? Dent Traumatol 21:276–280. https://doi.org/10.1111/j.1600-9657.2005.00354.x
Knapik JJ, Marshall SW, Lee RB, Darakjy SS, Jones SB, Mitchener TA, delaCruz GG, Jones BH. (2007) Mouthguards in sport activities: history, physical properties and injury prevention effectiveness. Sports Med Auckl NZ 37:117–144. https://doi.org/10.2165/00007256-200737020-00003
Lang B, Pohl Y, Filippi A (2002) Knowledge and prevention of dental trauma in team handball in Switzerland and Germany. Dent Traumatol Off Publ Int Assoc Dent Traumatol 18:329–334. https://doi.org/10.1034/j.1600-9657.2002.00123.x
Lässing J, Falz R, Pökel C, Fikenzer S, Laufs U, Schulze A, Hölldobler N, Rüdrich P, Busse M (2020a) Effects of surgical face masks on cardiopulmonary parameters during steady state exercise. Sci Rep 10:22363. https://doi.org/10.1038/s41598-020-78643-1
Lässing J, Schulze A, Falz R, Kwast S, Busse M (2020b) A randomized crossover study on the effects of a custom-made mouthguard on cardiopulmonary parameters and cortisol differences in a validated handball specific course. Injury. https://doi.org/10.1016/j.injury.2020.09.054
Lässing J, Schulze A, Kwast S, Falz R, Vondran M, Schröter T, Borger M, Busse M (2020c) Effects of custom-made mouthguards on cardiopulmonary exercise capacity. Int J Sports Med 41:1–8. https://doi.org/10.1055/a-1236-3814
Lee HP, Wang DY (2011) Objective assessment of increase in breathing resistance of N95 respirators on human subjects. Ann Occup Hyg 55:917–921. https://doi.org/10.1093/annhyg/mer065
Louhevaara VA (1984) Physiological effects associated with the use of respiratory protective devices. A review. Scand J Work Environ Health 10:275–281. https://doi.org/10.5271/sjweh.2327
Melissant CF, Lammers JW, Demedts M (1998) Relationship between external resistances, lung function changes and maximal exercise capacity. Eur Respir J 11:1369–1375
Mihalik JP, McCaffrey MA, Rivera EM, Pardini JE, Guskiewicz KM, Collins MW, Lovell MR (2007) Effectiveness of mouthguards in reducing neurocognitive deficits following sports-related cerebral concussion. Dent Traumatol Off Publ Int Assoc Dent Traumatol 23:14–20. https://doi.org/10.1111/j.1600-9657.2006.00488.x
Morales J, Buscà B, Solana-Tramunt M, Miró A (2015) Acute effects of jaw clenching using a customized mouthguard on anaerobic ability and ventilatory flows. Hum Mov Sci 44:270–276. https://doi.org/10.1016/j.humov.2015.09.008
Newsome PR, Tran DC, Cooke MS (2001) The role of the mouthguard in the prevention of sports-related dental injuries: a review. Int J Paediatr Dent 11:396–404. https://doi.org/10.1046/j.0960-7439.2001.00304.x
Petrović M, Kühl S, Šlaj M, Connert T, Filippi A (2016) Dental and general trauma in team handball. Swiss Dent J 126:682–686
Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B, Lonsdorfer J (2001) Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 85:202–207. https://doi.org/10.1007/s004210100458
Ryan KL, Cooke WH, Rickards CA, Lurie KG, Convertino VA (2008) Breathing through an inspiratory threshold device improves stroke volume during central hypovolemia in humans. J Appl Physiol Bethesda Md 1985 104:1402–1409. https://doi.org/10.1152/japplphysiol.00439.2007
Schulze A, Kwast S, Busse M (2019a) Influence of mouthguards on physiological responses in rugby. Sports Med Int Open 03:E25–E31. https://doi.org/10.1055/a-0891-7021
Schulze A, Kwast S, Busse M (2019b) Effects of a vented mouthguard on performance and ventilation in a basketball field setting. J Sports Sci Med 18:384–385
Schulze A, Laessing J, Kwast S, Busse M (2020) Influence of a vented mouthguard on physiological responses in handball. J Strength Cond Res 34:2055–2061. https://doi.org/10.1519/JSC.0000000000002596
Siebenmann C, Rasmussen P, Sørensen H, Zaar M, Hvidtfeldt M, Pichon A, Secher NH, Lundby C (2015) Cardiac output during exercise: a comparison of four methods. Scand J Med Sci Sports 25:e20–e27. https://doi.org/10.1111/sms.12201
von Arx T, Flury R, Tschan J, Buergin W, Geiser T (2008) Exercise capacity in athletes with mouthguards. Int J Sports Med 29:435–438. https://doi.org/10.1055/s-2007-965341
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JL and MB conceived and designed the research. JL and RF conducted the experiments. JL and RF analyzed the data. JL wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethics approval
Reference number 445-15-21122015.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Communicated by Philip D. Chilibeck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lässing, J., Falz, R., Schulze, A. et al. Decreased exercise capacity in young athletes using self-adapted mouthguards. Eur J Appl Physiol 121, 1881–1888 (2021). https://doi.org/10.1007/s00421-021-04659-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04659-8