Abstract
Purpose
To investigate (1) whether maximal stroke volume (SVmax) occurs at submaximal exercise intensities, (2) sex differences in SVmax once fat-free mass (FFM) has been controlled for, and, (3) the contribution of concurrent changes in FFM and SVmax to the sex-specific development of peak oxygen uptake \( \left( {{\dot{\text{V}}\text{O}}_{2} } \right) \).
Methods
The peak \( {\dot{\text{V}}\text{O}}_{2} \) s of 61 (34 boys) 11–12-year-olds were determined and their SV determined during treadmill running at 2.28 and 2.50 m s−1 using carbon dioxide rebreathing. The SVmax and peak \( {\dot{\text{V}}\text{O}}_{2} \) of 51 (32 boys) students who volunteered to be tested treadmill running at 2.50 m s−1 on three annual occasions were investigated using multilevel allometric modelling. The models were founded on 111 (71 from boys) determinations of SVmax, FFM, and peak \( {\dot{\text{V}}\text{O}}_{2} \).
Results
Progressive increases in treadmill running speed resulted in significant (p < 0.01) increases in \( {\dot{\text{V}}\text{O}}_{2} \), but SV levelled-off with nonsignificant (p > 0.05) changes within ~ 2–3%. In the multilevel models, SVmax increased proportionally to FFM0.72 and with FFM controlled for, there were no significant (p > 0.05) sex differences. Peak \( {\dot{\text{V}}\text{O}}_{2} \) increased with FFM but after adjusting for FFM0.98, a significant (p < 0.05) sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \) remained. Introducing SVmax to the multilevel model revealed a significant (p < 0.05), but small additional effect of SVmax on peak \( {\dot{\text{V}}\text{O}}_{2} \).
Conclusions
Fat-free mass explained sex differences in SVmax, but with FFM controlled for, there was still a ~ 5% sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \). SVmax made a modest additional contribution to explain the development of peak \( {\dot{\text{V}}\text{O}}_{2} , \) but there remained an unresolved sex difference of ~ 4%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peak oxygen uptake (\( {\dot{\text{V}}\text{O}}_{2} \)), the highest \( {\dot{\text{V}}\text{O}}_{2} \) elicited during an incremental exercise test to exhaustion, is internationally recognized as the criterion measure of youth cardiorespiratory fitness. Laboratory-based studies of boys’ peak \( {\dot{\text{V}}\text{O}}_{2} \) date back over 80 years (Robinson 1938) and data on both sexes have been available for over 65 years (Åstrand 1952), but the role of concurrent changes in morphological and physiological variables in the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) during growth and maturation remains to be fully elucidated (Armstrong and McManus 2017).
The extant literature consists largely of cross-sectional studies and the interpretation of data has been clouded by inappropriate ratio scaling of peak \( {\dot{\text{V}}\text{O}}_{2} \) with body mass (Welsman and Armstrong 2019). Allometric scaling of peak \( {\dot{\text{V}}\text{O}}_{2} \) data has enhanced understanding of youth cardiorespiratory fitness (Armstrong et al. 1998; Loftin et al. 2016; Welsman et al. 1996), but cross-sectional studies only provide a ‘snapshot’ of a continuous process. There are few rigorously analysed longitudinal studies, but the emergence (Aitkin et al. 1981), development (Rasbash et al. 2018), and application (Nevill et al. 1998) of multilevel allometric modelling to paediatric exercise physiology has provided new insights into the development of cardiorespiratory fitness. Using multilevel allometric modelling, the effects of age, maturity status, body size, body composition, and physiological covariates can be partitioned concurrently within an allometric framework to provide a sensitive interpretation of the development of peak \( {\dot{\text{V}}\text{O}}_{2} \). To our knowledge, no appropriate analysis including both morphological and physiological variables has been published.
Recent multilevel allometric models of large datasets have demonstrated the influence of age- and maturity status-driven concurrent changes in body mass and fat-free mass (FFM) on the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) (Armstrong and Welsman 2019a, b). Longitudinal analyses of 1057 treadmill determinations of 10–18-year-olds’ peak \( {\dot{\text{V}}\text{O}}_{2} \) showed that in both sexes with age and body mass controlled for, maturity status made a positive contribution to explaining peak \( {\dot{\text{V}}\text{O}}_{2} \). When FFM was introduced to the multilevel allometric models, the effects of maturity status became nonsignificant (p > 0.05) and the models provided a significantly (p < 0.05) better statistical fit to the data. The authors argued that in both sexes, the influence of maturity status on peak \( {\dot{\text{V}}\text{O}}_{2} \) was largely evidenced through maturation-driven changes in FFM (Armstrong and Welsman 2019a). These findings were confirmed in multilevel allometric modelling analyses of 320 cycle ergometer-determinations of 11–16-year-olds’ peak \( {\dot{\text{V}}\text{O}}_{2} \) (Armstrong and Welsman 2019b). However, regardless of exercise modality and with age, maturity status, and FFM controlled for, an unexplained sex difference remained with boys’ peak \( {\dot{\text{V}}\text{O}}_{2} \) significantly (p < 0.05) higher than that of girls and the difference increasing with age (Armstrong and Welsman 2019a, b).
In healthy youth, pulmonary ventilation does not limit peak \( {\dot{\text{V}}\text{O}}_{2} \) (McManus and Armstrong 2017a) so if the unexplained sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \) lies in its physiological components, cardiovascular variables are the prime candidates. The Fick equation describes \( {\dot{\text{V}}\text{O}}_{2} \) as the product of arteriovenous oxygen difference (A-VO2 diff) and cardiac output (\( {\dot{\text{Q}}} \)), with \( {\dot{\text{Q}}} \) a function of heart rate (HR) and stroke volume (SV). Methods of rigorously determining the HR and peak \( {\dot{\text{V}}\text{O}}_{2} \) of children and adolescents are well documented (Falk and Dotan 2019; Jones 1997; McManus and Armstrong 2017b), but understanding of cardiovascular responses to exercise, particularly at peak \( {\dot{\text{V}}\text{O}}_{2} \) is limited by ethical and methodological challenges. No direct measurements of youth A-VO2 diff, \( {\dot{\text{Q}}} \), or SV at peak \( {\dot{\text{V}}\text{O}}_{2} \) have been reported and there are, therefore, no ‘gold standard’ reference values as data from indirect methods of estimating A-VO2 diff, \( {\dot{\text{Q}}} \), and SV can only be compared within methodologies (Rowland 2017). Nevertheless, several methods of estimating children’s \( {\dot{\text{Q}}} \) during exercise have been developed and carbon dioxide (CO2) rebreathing, Doppler echocardiography, and bioimpedance cardiography have proved to be safe, non-invasive, repeatable, and reliable methods for estimating \( {\dot{\text{Q}}} \) and, therefore, A-VO2 diff, and SV in paediatric exercise studies (Patterson et al. 1982; Warburton and Bredin 2017; Welsman et al. 2005).
HR and A-VO2 diff at peak \( {\dot{\text{V}}\text{O}}_{2} \) have been consistently reported to be independent of age, body size, body composition, and sex during childhood and early adolescence (Armstrong and McManus 2017; Obert et al. 2003; Rowland 2017). SV is, therefore, the only cardiovascular factor differentiating peak \( {\dot{\text{V}}\text{O}}_{2} \) in girls and boys. During progressive upright exercise, SV initially increases and then plateaus at ~ 50% of peak \( {\dot{\text{V}}\text{O}}_{2} \) and remains essentially stable (within ± 5%) until exhaustion. This pattern is one of the most consistently observed responses in cardiac exercise physiology and has been demonstrated in healthy, untrained children and adolescents using a range of methodologies including CO2 rebreathing (Cunningham et al. 1994; Turley and Wilmore 1997), Doppler echocardiography (Nottin et al. 2002; Rowland et al. 2000b), and bioimpedance cardiography (McNarry et al. 2011, 2014). Values of exercise SV above ~ 50% of peak \( {\dot{\text{V}}\text{O}}_{2} \) have been reported to be highly predictive of maximal SV (SVmax) (Rowland et al. 1999a), characteristic of individuals as well as group means (Rowland et al. 2000b), and can be assumed to reflect SVmax (Rowland, 2005).
Direct comparisons of sex differences in SVmax are limited by a paucity of studies, small numbers of participants, data from girls being remarkably sparse, and studies predominantly focusing on pre-pubertal children. Studies consistently report boys’ absolute SVmax (i.e. in mL) to be higher than girls’ SVmax (Rowland et al. 2000a; Turley and Wilmore 1997; Vinet et al. 2003). In both sexes, SVmax increases with age and body size (Miyamura and Honda 1973; Nottin et al. 2002; Turley and Wilmore 1997) and to control for body size, SV is conventionally expressed in 1:1 ratio with body surface area (BSA) as the stroke index. Tanner (1949) unequivocally established that ratio scaling of cardiac data with BSA is fallacious and it has been demonstrated that with SV, the most appropriate scaling procedure is a curvilinear allometric model (Batterham et al. 1999; Rowland 2017). Once it has been allometrically scaled with BSA neither age nor maturity status appears to influence SV (McNarry et al. 2011; Rowland et al.1999b).
Stroke volume is, however, closely matched to metabolic demand and should be considered in relation to the active muscle mass rather than BSA. With the experimental challenges of determining active muscle mass, FFM has emerged as the recommended morphological variable with which to scale SV in paediatric exercise physiology. In cross-sectional studies, SV is best expressed in relation to FFM raised to an empirically derived allometric scaling exponent calculated from the participants in the study (Rowland 2017).
Only two studies have analysed children’s SVmax allometrically scaled with FFM, in both cases with 10–12-year-old pre-pubertal or pre-menarcheal children. Vinet et al. (2003) reported the sex difference in SVmax relative to FFM0.79 to be 4.8%, but not statistically significant (p > 0.05) and concluded that sex differences in peak \( {\dot{\text{V}}\text{O}}_{2} \) are a reflection of sex differences in body composition and not in cardiac functional capacity. Rowland et al. (2000a) reported the sex difference in SVmax relative to FFM0.84 to be 5.2%, but not statistically significant (p > 0.05). These authors, however, argued that despite not being statistically significant, the observed sex difference in SVmax in pre-pubertal children was small, but ‘real’ and suggested that sex differences in SVmax account for differences in peak \( {\dot{\text{V}}\text{O}}_{2} \) between girls and boys once FFM had been controlled for.
In summary, FFM has been demonstrated to exert a powerful influence on the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) but even with FFM controlled for, it is evident that there are still sex differences in peak \( {\dot{\text{V}}\text{O}}_{2} \) during childhood and adolescence (Armstrong and Welsman 2019a, b). SVmax appears to be the principal potential cardiovascular contributor to an explanation of sex differences in the development of peak \( {\dot{\text{V}}\text{O}}_{2} \). Current data interpretations are in conflict, but cross-sectional studies of pre-pubertal children provide limited insights into the development of SVmax and its influence on the development of peak \( {\dot{\text{V}}\text{O}}_{2} \). The purposes of the present studies are, therefore, to initially confirm that SV plateaus during progressive treadmill running above moderate exercise intensities and then to use multilevel allometric modelling to investigate in childhood and early adolescence (1) sex differences in SVmax once FFM has been controlled for, and, (2) the contribution of concurrent changes in FFM and SVmax to the sex-specific development of peak \( {\dot{\text{V}}\text{O}}_{2} \).
Methods
Participants
Students from local state schools participating in a longitudinal study of the development of cardiorespiratory fitness and short-term power output (Armstrong and Welsman 2019a, c) also volunteered to have their \( {\dot{\text{Q}}} \) determined. Some of the initial submaximal \( {\dot{\text{Q}}} \) data were published as the project progressed (Armstrong and Welsman 2002), but the present datasets have not previously been brought together for analysis and the contribution of SVmax to the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) has not been addressed. In Study 1, 61 (34 boys) students completed the exercise tests while treadmill running at 2.22 m s−1 and 2.50 m s−1 with 35 students (27 boys) completing a third stage of treadmill running at 2.78 m s−1. For Study 2, 51 (32 boys) of the students agreed to be tested on three annual occasions while running on a treadmill at 2.50 m s−1.
Experimental procedures
Determination of resting variables
Participants were well habituated to the laboratory environment, to the laboratory personnel, and to the experimental procedures. Age was computed from date of birth and date of test. Anthropometric measures were taken as described by the International Biological Programme (Weiner and Lourie 1981) and apparatus was calibrated according to the manufacturers’ instructions. Body mass was assessed using Avery balance scales (Avery, Birmingham, UK), stature was measured using a Holtain stadiometer (Holtain, Crmych, Dyfed, UK), and skinfold thicknesses over the triceps and subscapular regions were measured using Holtain skinfold calipers. Maturity status was visually assessed by the Research Centre nurse using the indices for pubic hair (PH) development described by Tanner (1962). FFM was estimated from skinfolds, body mass, and maturity status using the youth-specific equations developed by Slaughter et al. (1988). Haemoglobin (Hb) concentration was determined as the mean value from duplicate fingertip blood samples which were immediately assayed using a Hemo Cue photometer (Clandon Scientific, Farnborough, UK).
Determination of exercise variables
Participants attended the Research Centre on two consecutive mornings to complete the required exercise protocols. Peak \( {\dot{\text{V}}\text{O}}_{2} \) was determined on day 1 and at a similar time, the following morning SV and associated cardiovascular variables were determined. All exercise tests were preceded by a standardized warm-up. Peak \( {\dot{\text{V}}\text{O}}_{2} \) was determined during a discontinuous, incremental exercise test to voluntary exhaustion on a motorized treadmill (Woodway, Cranlea Medical, Birmingham, UK). HR was monitored using an electrocardiograph (Rigel, Morden, UK) and expired respiratory gases were monitored continuously using an Oxycon Sigma on-line gas-analysis system (Cranlea Medical) which was calibrated prior to each test using gases of verified concentration and an appropriate range of flow rates using a Hans Rudolph calibration syringe (Cranlea Medical). The tests began at a treadmill belt speed of 1.94 m s−1 (7 km h−1) which was increased by 0.28 m s−1 (1 km h−1) every 3 min until a speed of 2.78 m s−1 (10 km h−1) was reached. Subsequently, belt speed was held constant and the gradient was incrementally increased by 2.5% every 3 min until voluntary exhaustion. A 1-min rest period separated the exercise stages. The highest 30 s \( {\dot{\text{V}}\text{O}}_{2} \) attained was accepted as a maximal index if clear signs of intense exertion (e.g. hyperpnea, facial flushing unsteady gait, profuse sweating) were demonstrated and supported by a respiratory exchange ratio greater than 1.00 and a HR which was levelling-off over the final stages of the test at a value within 5% of the mean maximal HRs (i.e. 202 beats min−1) we have previously reported for large groups of similar-aged young people using the same test protocol (Armstrong et al. 1991). All participants reported in this study satisfied these criteria.
On the following morning, using the same apparatus including the \( {\dot{\text{Q}}} \) determination facility of the Oxycon Sigma \( {\dot{\text{V}}\text{O}}_{2} \), HR, and \( {\dot{\text{Q}}} \) were determined during the final minute of 3 min treadmill running stages at 2.22 m s−1 (8 km h−1) and 2.50 m s−1 (9 km h−1) interspersed with a 1-min rest period. Following a further 1-min rest, 35 students (27 boys) satisfactorily completed a third stage running at 2.78 m s−1 (10 km h−1). \( {\dot{\text{Q}}} \) was determined indirectly using the CO2 rebreathing technique in accordance with the methodology recommended by Jones (1997). The partial pressure of CO2 in arterial blood (PaCO2) was estimated from the end tidal CO2. The partial pressure of mixed venous CO2 (PvCO2) was estimated from the rebreathing equilibrium. The downstream correction was applied to the partial pressure of the equilibration CO2 to adjust for alveolar to blood partial pressure differences. The CO2 content of venous and arterial blood was calculated from PvCO2 and PaCO2 using the McHardy curve adjusted for the effect of individual differences in haemoglobin (McHardy et al. 1967). The volume of gas in the rebreathing bag was calculated to be 1.5 times the mean of three previous tidal breaths and the bag CO2 concentration, which varied from 9 to 13%, was calculated based on the \( {\dot{\text{V}}\text{O}}_{2} \) and the end tidal partial pressure of CO2. The size of the rebreathing bag was selected individually to accommodate the gas volume, but without being so large as to prevent appropriate CO2 equilibrium. Only tests which demonstrated a CO2 equilibrium were included in the data (Jones 1997). Individual SVs were calculated by dividing \( {\dot{\text{Q}}} \) with HR.
Data analysis
Study 1 Data were stored and analysed using SPSS version 25 (IBM, SPSS statistics, Portsmouth, UK). Descriptive data (means and standard deviations) were computed, between-sex differences were explored, and within-sex differences across the exercise stages were examined using ANOVA and paired t tests. Significance was set at p < 0.05.
Study 2 To describe relationships between FFM, SVmax, and peak \( {\dot{\text{V}}\text{O}}_{2} \), data were graphed and Pearson product moment correlation coefficients were computed. Data were analysed using multilevel regression modelling (MLWin v3.02, Centre for Multilevel Modeling, University of Bristol, UK). Longitudinal changes in (1) SVmax with FFM and age, and, (2) in peak \( {\dot{\text{V}}\text{O}}_{2} \) with FFM, SVmax, and age were analysed using the multiplicative, allometric approach introduced by Nevill et al. (1998) as follows:
where y = SVmax or peak \( {\dot{\text{V}}\text{O}}_{2} \).
Log transformation linearizes the model as in the following equation forming the starting point for analyses:
where y = SVmax or peak \( {\dot{\text{V}}\text{O}}_{2} \).
All parameters were fixed with the exception of the constant (a) which was allowed to vary randomly at level 2 (between individuals) and the multiplicative error ratio (ε) which also varied randomly at level 1 (within individuals) as denoted by the subscripts i (level 1 variation) and j (level 2 variation). Age was centred on the group mean. Other factors associated with the dependent variable were explored either as additional covariates or through the calculation of dummy variables (setting the boys’ constant as baseline and investigating any departure from this for girls) or interaction terms allowing for different relationships between covariates and sex to be examined.
Parameter estimates were considered significant (p < 0.05) where their value exceeded 2 × the standard error (SE). Where more than one model was investigated, a comparison of the goodness of fit of the different models was obtained from the change in the deviance statistic (− 2 × log-likelihood) with reference to the number of fitted parameters. The model with the smallest log-likelihood reflects that with the best fit for the same number of fitted parameters. Additional parameters contribute to improved fit from the change in the log-likelihood according to a χ² statistic for additional degrees of freedom added.
Results
Study 1 Descriptive data are presented in Table 1. Maturity status ranged from 9 boys and 4 girls pre-pubertal (at stage 1 for PH) to 13 boys and 9 girls at stage 2, 9 boys and 11 girls at stage 3, 3 boys and 2 girls at stage 4, and 1 girl at stage 5. There were no significant (p > 0.05) sex differences in age, stature, body mass, sum of two skinfolds, FFM, HR at peak \( {\dot{\text{V}}\text{O}}_{2} \), or Hb concentration, but boys had a significantly (p < 0.01) higher mean peak \( {\dot{\text{V}}\text{O}}_{2} \) than girls.
Table 2 summarizes exercise responses during consecutive 3 min treadmill running stages at 2.22 and 2.50 m s−1. In both sexes, there were significant (p < 0.01) increases in \( {\dot{\text{V}}\text{O}}_{2} \), % peak \( {\dot{\text{V}}\text{O}}_{2} \) and HR with progressively increasing treadmill speed, but no significant (p > 0.05) change in SV (change: boys ~ 2%; girls ~ 3%). A subsample of this group continued with a further exercise bout running at 2.78 m s−1. For the 27 boys, % peak \( {\dot{\text{V}}\text{O}}_{2} \) significantly (p < 0.01) increased to 78.7 ± 6.4% with a nonsignificant (p > 0.05) ~ 3% change in SV to 82.6 ± 11.7 mL. For the 8 girls who also exercised at a treadmill speed of 2.78 m s−1, % peak \( {\dot{\text{V}}\text{O}}_{2} \) significantly (p < 0.01) increased to 89.4 ± 7.7% with a nonsignificant (p > 0.05) ~ 3% change in SV to a mean value of 71.8 ± 7.6 mL recorded.
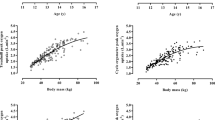
Study 2 Figure 1 illustrates the sex-specific relationships between SVmax and FFM. Figure 2 illustrates the sex-specific relationships between peak \( {\dot{\text{V}}\text{O}}_{2} \) and FFM, and peak \( {\dot{\text{V}}\text{O}}_{2} \) and SVmax. Significant (p < 0.01) correlation coefficients between SVmax and FFM were r = 0.78 and r = 0.62 for boys and girls, respectively. Peak \( {\dot{\text{V}}\text{O}}_{2} \) was significantly (p < 0.01) correlated with FFM (boys, r = 0.96; girls, r = 0.89) and with SVmax (boys, r = 0.78; girls, r = 0.74).
Maximal stroke volume in relation to fat-free mass by sex. Data are from 111 (71 boys) determinations of maximal stroke volume and fat-free mass. Fat-free mass is estimated from youth-specific equations (Slaughter et al. 1988)
Peak oxygen uptake in relation to maximal stroke volume and fat-free mass by sex. Data are from 111 (71 boys) determinations of peak oxygen uptake, maximal stroke volume, and fat-free mass. Fat-free mass is estimated from youth-specific equations (Slaughter et al. 1988)
In contrast to traditional methods that require a complete longitudinal dataset, both the number of observations per individual and the temporal spacing of the observations can vary within a multilevel analysis. The multilevel analyses presented herein are founded on 111 (71 from boys) determinations of FFM, SVmax, and peak \( {\dot{\text{V}}\text{O}}_{2} \). Table 3 presents multilevel allometric models for SVmax with FFM. In model 3.1, loge FFM was revealed as a significant (p < 0.05) explanatory variable with SVmax increasing proportionally to FFM0.72. With FFM controlled for, there were no significant sex differences in SVmax. Models 3.2 and 3.3 describe very similar models for SVmax by sex. In boys, the FFM exponent was 0.72 as in the combined sex model and in girls, the FFM exponent was a similar 0.68. Age and maturity status were not significant (p > 0.05) factors in any model.
Table 4 describes the multilevel allometric models for peak \( {\dot{\text{V}}\text{O}}_{2} \). Model 4.1 shows peak \( {\dot{\text{V}}\text{O}}_{2} \) to increase in almost direct proportion with FFM, but after adjusting for FFM, girls’ peak \( {\dot{\text{V}}\text{O}}_{2} \) was ~ 5% lower than boys’ peak \( {\dot{\text{V}}\text{O}}_{2} \). Introducing loge SVmax as an additional covariate in Model 4.2 yielded a significant (p < 0.05) parameter estimate of 0.12 (SE .05) showing an additional effect of SVmax on peak \( {\dot{\text{V}}\text{O}}_{2} \) and providing a model with a significantly (p < 0.05) better statistical fit. However, a significant (p < 0.05) ~ 4% sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \) remained. In boys (Model 4.3), once FFM had been controlled for, SVmax was not a significant (p > 0.05) additional explanatory variable for peak \( {\dot{\text{V}}\text{O}}_{2} \). For girls (Model 4.4), both FFM and SVmax were significant explanatory variables (p < 0.05) with exponents for FFM of 0.93 (SE .09) and SVmax of 0.18 (SE 0.08). Age, maturity status, and Hb concentration were not significant (p > 0.05) factors in any model.
Discussion
Study 1 empirically confirmed that during late childhood and early adolescence, SV plateaus during progressive treadmill running. Despite an increase in treadmill running speed which induced significant increases in HR, \( {\dot{\text{V}}\text{O}}_{2} \), and % peak \( {\dot{\text{V}}\text{O}}_{2} \) in both sexes, SV did not rise but levelled-off with values within ~ 2–3%. The underlying physiology suggests that at the onset of progressive upright exercise, the joint effects of arteriolar vasodilation and the pumping action of the skeletal muscles in the legs mobilize the blood pooled in the legs and increase the venous return to the heart. SV increases from rest by ~ 30–40% before levelling-off and remaining stable with further increases in exercise intensity (Rowland et al. 2000b; Rowland and Unnithan 2013: Tschakovsky et al. 1996). The stability of SV is maintained by the regulation of HR which rises in parallel with the venous return not only to increase \( {\dot{\text{Q}}} \) but also to ‘defend’ the optimal SV through to peak \( {\dot{\text{V}}\text{O}}_{2} \) (Rowland 2017; Winsley 2007).
The failure of SV to increase further once progressive exercise reaches ~ 50% of peak \( {\dot{\text{V}}\text{O}}_{2} \) allows the CO2 rebreathing technique to be used in paediatric studies to estimate SVmax. Both the present authors’ observations and the data support the view that children and adolescents find rebreathing CO2 to be disagreeable, particularly when exercising near their peak \( {\dot{\text{V}}\text{O}}_{2} \) (Driscoll et al. 1989; Washington 1993; Winsley 2007). Notably, increasing the treadmill belt speed to elicit ~ 90% peak \( {\dot{\text{V}}\text{O}}_{2} \) reduced participant compliance which resulted in only 8 (~ 30%) girls being willing or able to complete the stage. However, even in these girls, SV did not significantly (p > 0.05) change (~ 3%) with an exercise step change from ~ 78 to 89% of peak \( {\dot{\text{V}}\text{O}}_{2} \). 27 boys (~ 79%) satisfactorily completed the third stage which was at ~ 79% of peak \( {\dot{\text{V}}\text{O}}_{2} \) with a mean SV which was within 1% of that at ~ 68% of peak \( {\dot{\text{V}}\text{O}}_{2} \). These data confirm the extant literature (e.g. Rowland 2005) that submaximal SV, in the present case above ~ 67% (boys) and ~ 77% (girls) of peak \( {\dot{\text{V}}\text{O}}_{2} \), can be assumed to reflect SVmax.
The vast majority of studies of paediatric exercise SV have involved cycle ergometry and, with the range of techniques employed to estimate SVmax, absolute values are not directly comparable across studies (Warburton and Bredin 2017). Within study, sex differences in absolute SVmax are, however, consistent and the % sex differences in SVmax in the present study (~ 6–7%) are in remarkable agreement with those reported elsewhere for children and early adolescents (~ 5–7%) (Rowland et al. 2000a; Turley and Wilmore 1997; Vinet et al. 2003).
SVmax essentially depends on cardiac size and function and although empirical data are sparse, the extant literature suggests that sex differences in SVmax reflect differences in cardiac dimensions (Turley and Wilmore 1997; Vinet et al. 2003). Cardiac dimensions are closely associated with FFM and a potential relationship between skeletal and cardiac muscularity has been suggested (Batterham et al. 1999; George et al. 1991). Vinet et al. (2003) reported that in pre-pubertal children with FFM allometrically controlled for, sex differences in left ventricular dimensions and left ventricular mass were no longer significant (p > 0.05). Figure 1 illustrates the relationship between FFM and SVmax supported by significant (p < 0.01) correlations of r = 0.78 and r = 0.62, in boys and girls, respectively.
Model 3.1 (Table 3) shows that SVmax increases in proportion to FFM0.72 and with FFM controlled for, there was no significant (p > 0.05) sex difference in SVmax. This is in agreement with earlier cross-sectional studies of similar-aged children which also observed sex differences in SVmax to be not significant (p > 0.05) following allometric normalization with FFM (Rowland et al. 2000a; Vinet et al. 2003). Moreover, these authors reported similar FFM exponents of 0.84 and 0.79, respectively, both of which fall within the 95% confidence limits of the present FFM exponent of 0.72 (0.07). Sex-specific models of SVmax were similar with FFM exponents of 0.72 (Model 3.2, boys) and 0.69 (Model 3.3, girls). Once FFM had been controlled for, age and maturity status were not significant (p > 0.05) explanatory variables in any model, but this may have been influenced by the limited range of ages and population of stages in maturity status in the present study of 11–13-year-olds. There are no longitudinal studies with which to compare the present data.
The strong influence of FFM on the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) across the age range 10–18 years is well documented and has been largely attributed to increases in muscle mass enhancing total muscle \( {\dot{\text{V}}\text{O}}_{2} \) and venous return during exercise (Armstrong and Welsman 2019a). In mid- and late-adolescence, a marked increase in FFM is strongly influenced by the timing and tempo of maturation, particularly in boys (Armstrong 2019; Baxter-Jones et al. 2003). Figure 2 illustrates that even with children and early adolescents, there are strong relationships between peak \( {\dot{\text{V}}\text{O}}_{2} \) and FFM with similar significant (p < 0.01) correlations to those observed by Armstrong and Welsman (2019a) over an age range of 10–18 years in both boys (r = 0.96 vs r = 0.94) and girls (r = 0.89 vs r = 0.87). Model 4.1 (Table 4) shows a significant (p < 0.05) sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \) of ~ 5% once FFM had been controlled for, with age having no additional significant effect. Whereas, in 10–18-year-olds with FFM controlled for, age had a significant (p < 0.05) positive effect on peak \( {\dot{\text{V}}\text{O}}_{2} \) supplemented with a significant positive age by sex interaction which resulted in a larger sex difference of ~ 10% (Armstrong and Welsman 2019a).
Figure 2 shows the relationship between peak \( {\dot{\text{V}}\text{O}}_{2} \) and SVmax in both sexes with significant (p < 0.01) correlations in boys and girls of r = 0.78 and r = 0.74, respectively. The introduction of SVmax into Model 4.2 resulted in a significant (p < 0.05) positive effect on peak \( {\dot{\text{V}}\text{O}}_{2} \), a small reduction to ~ 4% in the sex difference, and a significantly (p < 0.05) better statistical fit for the combined sex data. Model 4.4 of the girls’ data shows a similar pattern with SVmax having a moderate, but significant (p < 0.05) effect on peak \( {\dot{\text{V}}\text{O}}_{2} \). On the other hand, in boys, the effect of SVmax on peak \( {\dot{\text{V}}\text{O}}_{2} \) was small and not significant (p > 0.05) once FFM had been controlled for as showed in Model 4.3. Any effects of SVmax on boys’ peak \( {\dot{\text{V}}\text{O}}_{2} \) appear to be masked by and reflected in the increasing FFM in boys, even during early adolescence.
Fat-free mass is the most powerful morphological influence on the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) in both sexes and accounts for much of the increasing sex difference in the development of aerobic fitness during youth. The present data suggest that with FFM controlled for, the sex difference in the peak \( {\dot{\text{V}}\text{O}}_{2} \) of children and early adolescents falls from ~ 15 to 5%. SVmax makes a statistically significant (p < 0.05) contribution to explain the development of peak \( {\dot{\text{V}}\text{O}}_{2} , \) but an unexplained sex difference remains. Why with FFM and SVmax controlled for there remains a ~ 4% sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \) is unresolved.
The extant literature provides unequivocal evidence of no sex difference in HRmax (Armstrong and McManus 2017) and strongly suggests no sex differences in maximal A-VO2 diff (Rowland 2017), although in this case, there are some conflicting data (Winsley et al. 2009). There were no sex differences in Hb concentration in the present studies, but even when sex differences in Hb are apparent as in late adolescence any effect on sex differences in peak \( {\dot{\text{V}}\text{O}}_{2} \) remains to be proven (Armstrong and Welsman 2001). A poorer matching of muscle oxygen delivery to oxygen utilization in 9–10-year-old girls than similar-aged boys has been reported from a study using near infra-red spectroscopy to estimate microcirculatory changes in deoxygenated Hb and myoglobin (McNarry et al. 2015), but this has not been confirmed. Methodological and ethical challenges leave it unclear whether there are meaningful sex differences in the ratio of active muscle mass to FFM, or in muscle structure, or in aerobic enzyme activity, or in muscle fibre types, or in muscle activation (Armstrong et al. 2017; Dotan et al. 2012; Malina et al. 2004). For the physiological mechanisms underlying sex differences in the development of aerobic fitness to be fully elucidated further development and application of non-invasive technology to developmental exercise physiology is required.
Strengths and limitations of the studies
Limitations to the present study potentially include the methodology of estimating SV at ~ 83% (girls) and ~ 74% (boys) of peak \( {\dot{\text{V}}\text{O}}_{2} \) and extrapolating it to represent SVmax. However, the validity of extrapolating SV values from submaximal exercise to SVmax is well documented and evidenced using a range of techniques, the data presented herein clearly demonstrate the SV plateau phenomenon (~ 2–3% change) with these participants, and we analysed data as close to peak \( {\dot{\text{V}}\text{O}}_{2} \) as reasonable for the young participants, many of whom found CO2 rebreathing unpleasant at high exercise intensities. The estimation rather than the direct measurement of FFM can be criticised, but the methodology is well established in paediatric exercise physiology (Slaughter et al. 1988) and ‘direct’ measures of body fat of the same young people have recently been shown to vary widely across laboratory techniques (Ferri-Morales et al. 2018). Moreover, FFM includes tissues not involved in exercise and ideally active muscle mass would be directly determined on each test occasion. This is not currently feasible in paediatric exercise studies involving over 120 multiple assessments. Another limitation is that although the study was longitudinal, it only covered a 2 year period. On the other hand, a unique strength of the study lies in the provision for the first time of longitudinal insights into the development of SVmax and its influence on peak \( {\dot{\text{V}}\text{O}}_{2} \) in healthy, untrained children and early adolescents. A major strength lies in the adoption of a multiplicative allometric modelling approach which allowed variables to be partitioned concurrently within an allometric framework to provide sensitive interpretations of the development of SVmax and peak \( {\dot{\text{V}}\text{O}}_{2} \).
Conclusions
Study 1 confirms that during progressive treadmill running, SV levels-off and reaches its maximum at submaximal intensities. Although there are no comparable longitudinal studies, Study 2 data are in accordance with cross-sectional analyses of children and early adolescents and demonstrate that there are no significant sex differences in SVmax once FFM has been appropriately controlled for. Multilevel allometric models demonstrate that FFM exerts a powerful influence on the development of peak \( {\dot{\text{V}}\text{O}}_{2} \) but even with FFM controlled for, there remains a ~ 5% sex difference. SVmax makes a modest additional contribution to explaining the development of peak \( {\dot{\text{V}}\text{O}}_{2} , \) but a residual ~ 4% sex difference in peak \( {\dot{\text{V}}\text{O}}_{2} \) remains unexplained.
Abbreviations
- A-VO2 diff:
-
Arteriovenous oxygen difference
- BSA:
-
Body surface area
- CO2 :
-
Carbon dioxide
- \( {\dot{\text{Q}}} \) :
-
Cardiac output
- FFM:
-
Fat-free mass
- Hb:
-
Haemoglobin
- HR:
-
Heart rate
- Max:
-
Maximal
- \( {\dot{\text{V}}\text{O}}_{2} \) :
-
Oxygen uptake
- PaCO2 :
-
Partial pressure of CO2 in arterial blood
- PvCO2 :
-
Partial pressure of mixed venous CO2
- PH:
-
Pubic hair
- SV:
-
Stroke volume
References
Aitkin M, Anderson D, Hinde J (1981) Statistical modelling of data on teaching styles. J Roy Stat Soc A 144:148–161
Armstrong N (2019) Development of the youth athlete. Routledge, Oxford, pp 5–26
Armstrong N, McManus AM (2017) Aerobic fitness. In: Armstrong N, van Mechelen W (eds) Oxford textbook of children’s sport and exercise medicine, 3rd edn. Oxford University Press, Oxford, pp 161–180
Armstrong N, Welsman JR (2001) Peak oxygen uptake in relation to growth and maturation in 11–17-year-old humans. Eur J Appl Physiol 85:546–551
Armstrong N, Welsman JR (2002) Cardiovascular responses to submaximal treadmill running in 11 to 13 year-olds. Acta Paediatr 91:126–131
Armstrong N, Welsman J (2019a) Sex-specific longitudinal modeling of youth aerobic fitness. Pediatr Exerc Sci 31:204–212
Armstrong N, Welsman J (2019b) Development of peak oxygen uptake from 11 to 16 years determined using both treadmill and cycle ergometry. Eur J Appl Physiol 119:801–812
Armstrong N, Welsman J (2019c) Sex-specific longitudinal modeling of short-term power in 11- to 18-year-olds. Med Sci Sports Exerc 51:1055–1063
Armstrong N, Williams J, Balding J, Gentle P, Kirby B (1991) Peak oxygen uptake of British children with reference to age, sex and sexual maturity. Eur J Appl Physiol 62:369–375
Armstrong N, Welsman JR, Kirby BJ (1998) Peak oxygen uptake and maturation in 12 year-olds. Med Sci Sports Exerc 30:165–169
Armstrong N, Barker AR, McManus AM (2017) Muscle metabolism during exercise. In: Armstrong N, van Mechelen W (eds) Oxford textbook of children’s sport and exercise medicine, 3rd edn. Oxford University Press, Oxford, pp 69–87
Åstrand PO (1952) Experimental studies of physical working capacity in relation to sex and age. Munksgaard, Copenhagen
Batterham AM, George KP, Whyte G, Sharma S, McKenna W (1999) Scaling cardiac structural data by body dimensions: a review of theory, practice and problems. Int J Sports Med 20:495–502
Baxter-Jones ADG, Mirwald RL, McKay HA, Bailey D (2003) A longitudinal analysis of sex differences in bone mineral accrual in healthy 8–19-year-old boys and girls. Ann Hum Biol 30:160–175
Cunningham DA, Paterson DH, Blimkie CJR, Donner AP (1994) Development of cardiorespiratory function in circumpubertal boys: a longitudinal study. J Appl Physiol 56:302–307
Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B (2012) Child-adult differences in muscle activation—a review. Pediatr Exerc Sci 24:2–21
Driscoll DJ, Staats BA, Beck KC (1989) Measurement of cardiac output in children during exercise: a review. Pediatr Exerc Sci 1:102–115
Falk B, Dotan R (2019) Measurement and interpretation of maximal aerobic power in children. Pediatr Exerc Sci. 31:144–151
Ferri-Morales A, Nascimento-Ferreira MV, Vlachopoulos D et al (2018) Agreement between standard body composition methods to estimate percentage of body fat in young male athletes. Pediatr Exerc Sci 30:402–410
George KP, Wolfe LA, Burggraf GW (1991) The ‘athletic heart syndrome’: a critical review. Sports Med 11:300–331
Jones NL (1997) Clinical exercise testing, 4th edn. WB Saunders Company, Philadelphia, pp 109–123 (150–161)
Loftin M, Sothern M, Abe T, Bonis M (2016) Expression of \( {\dot{\text{V}}\text{O}}_{2} \) peak in children and youth with special reference to allometric scaling. Sports Med 46:1451–1460
Malina RM, Bouchard C, Bar-Or O (2004) Growth, maturation and physical activity, 2nd edn. Human Kinetics, Champaign
McHardy GJR, Jones NL, Campbell EJM (1967) Graphical analysis of carbon dioxide transport during exercise. Clin Sci 32:289–298
McManus AM, Armstrong N (2017a) Pulmonary function. In: Armstrong N, van Mechelen W (eds) Oxford textbook of children’s sport and exercise medicine, 3rd edn. Oxford University Press, Oxford, pp 133–146
McManus AM, Armstrong N (2017b) Maximal oxygen uptake. In: Rowland TW (ed) Cardiopulmonary exercise testing in children and adolescents. Human Kinetics, Champaign, pp 79–93
McNarry MA, Welsman JR, Jones AM (2011) Influence of training and maturity status on the cardiopulmonary responses to ramp incremental cycle and upper body exercise in girls. J Appl Physiol 110:375–381
McNarry MA, Mackintosh KA, Stoedefalke K (2014) Longitudinal investigation of training status and cardiopulmonary responses in pre- and early-pubertal children. Eur J Appl Physiol 114:1573–1780
McNarry MA, Farr C, Middlebrooke A, Welford D, Breese B, Armstrong N, Barker AR (2015) Aerobic function and muscle deoxygenation dynamics during ramp exercise in children. Med Sci Sports Exerc 47:1877–1884
Miyamura M, Honda Y (1973) Maximum cardiac output related to sex and age. Jpn J Physiol 23:645–656
Nevill AM, Holder RL, Baxter-Jones A, Round JM, Jones DA (1998) Modelling developmental changes in strength and aerobic power in children. J Appl Physiol 84:963–970
Nottin S, Vinet A, Stecken N, Nguyen L-D, Ounissi F, Lecoq A-M, Obert P (2002) Central and peripheral cardiovascular adaptations during maximal cycle exercise in boys and men. Med Sci Sports Exerc 33:456–463
Obert P, Mandigout S, Nottin S, Vinet A, Nguyen L-D, Lecoq A-M (2003) Cardiovascular response to endurance training in children: effect of gender. Eur J Clin Invest 33:199–208
Patterson DH, Cunningham DA, Plyley MJ, Blimkie CJR, Donner A (1982) Reliability and reproducibility of cardiac output measurements in children. Eur J Appl Physiol 49:37–44
Rasbash J, Steele F, Browne WJ, Goldstein H (2018) A user’s guide to MLwiN Version 3.02. University of Bristol Centre for Multilevel Modelling, Bristol
Robinson S (1938) Experimental studies of physical fitness in relation to age. Arbeitsphysiologie 110:251–323
Rowland TW (2005) Children’s exercise physiology, 2nd edn. Human Kinetics, Champaign, pp 89–133
Rowland TW (2017) Cardiovascular function. In: Armstrong N, van Mechelen W (eds) Oxford textbook of children’s sport and exercise medicine, 3rd edn. Oxford University Press, Oxford, pp 147–159
Rowland T, Unnithan V (2013) Stroke volume dynamics during progressive exercise in healthy adolescents. Pediatr Exerc Sci 25:173–185
Rowland T, Goff D, Martel L, Ferrone L (1999a) Estimation of maximal stroke volume from submaximal values. Pediatr Exerc Sci 11:279
Rowland T, Miller K, Vanderburgh P, Goff D, Martel L, Ferrone L (1999b) Cardiovascular fitness in premenarchael girls and young women. Int J Sports Med 20:117–121
Rowland T, Goff D, Martel L, Ferrone L (2000a) Influence of cardiac functional capacity on gender differences in maximal oxygen uptake in children. Chest 17:629–635
Rowland T, Potts J, Potts T, Sandor G, Goff D, Ferrone L (2000b) Cardiac responses to progressive exercise in normal children: a synthesis. Med Sci Sports Exerc 32:253–259
Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA (1988) Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60:709–723
Tanner JM (1949) Fallacy of per-weight and per-surface area standards and their relation to spurious correlation. J Appl Physiol 2:1–15
Tanner JM (1962) Growth at adolescence, 2nd edn. Blackwell, Oxford, pp 28–39
Tschakovsky ME, Shoemaker JK, Hughson RL (1996) Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol 271:H1607–H1701
Turley KR, Wilmore JH (1997) Cardiovascular responses to submaximal exercise in 7- to 9-yr-old boys and girls. Med Sci Sports Exerc 29:824–832
Vinet A, Mandigout S, Nottin S, Nguyen LD, Lecoq A-M, Courteix D, Obert P (2003) Influence of body composition, hemoglobin concentration, and cardiac size and function on gender differences in maximal oxygen uptake in prepubertal children. Chest 124:1494–1499
Warburton DER, Bredin SSD (2017) Cardiac output measurement techniques. In: Rowland TW (ed) Cardiopulmonary exercise testing in children and adolescents. Human Kinetics, Champaign, pp 107–118
Washington RL (1993) Measurement of cardiac output. In: Rowland TW (ed) Pediatric laboratory exercise testing. Human Kinetics, Champaign, pp 131–140
Weiner JS, Lourie JA (1981) Practical human biology. Academic Press, London, pp 33–51
Welsman J, Armstrong N (2019) Interpreting aerobic fitness in youth: the fallacy of ratio scaling. Pediatr Exerc Sci 31:184–190
Welsman JR, Armstrong N, Kirby BJ, Nevill AM, Winter EM (1996) Scaling peak \( {\dot{\text{V}}\text{O}}_{2} \) for differences in body size. Med Sci Sports Exerc 28:259–265
Welsman JR, Bywater K, Farr C, Welford D, Armstrong N (2005) Reliability of peak \( {\dot{\text{V}}\text{O}}_{2} \) and maximal cardiac output assessed using thoracic bioimpedance in children. Eur J Appl Physiol 94:228–234
Winsley RJ (2007) Cardiovascular function. In: Armstrong N (ed) Paediatric exercise physiology. Churchill Livingstone, Edinburgh, pp 139–160
Winsley RJ, Fulford J, Roberts AC, Welsman JR, Armstrong N (2009) Sex difference in peak oxygen uptake in prepubertal children. J Sci Med Sport 12:647–657
Acknowledgements
We gratefully acknowledge the commitment of the participants, the logistic support of Exeter schools, and the technical assistance of the Children’s Health and Exercise Research Centre team.
Funding
This study was funded by the British Heart Foundation and the Darlington Trust.
Author information
Authors and Affiliations
Contributions
NA and JW jointly conceived and designed the research, led the research team, and analysed the data. Both authors contributed to the drafting of the manuscript, both authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study and from their legal guardians.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Armstrong, N., Welsman, J. Multilevel allometric modelling of maximal stroke volume and peak oxygen uptake in 11–13-year-olds. Eur J Appl Physiol 119, 2629–2639 (2019). https://doi.org/10.1007/s00421-019-04241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04241-3