Abstract
Purpose
The aim of this study was to examine whether differences in vascular responsiveness associated with training status would be more prominent in the trained limb (leg) than in the untrained limb (arm) microvasculature.
Methods
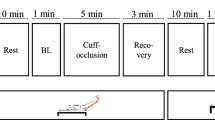
Thirteen untrained (26 ± 5 year) and twelve trained (29 ± 4 year) healthy men were submitted to a vascular occlusion test (VOT) (2 min baseline, 5 min occlusion, and 8 min re-oxygenation). The oxygen saturation signal (StO2) was assessed using near-infrared spectroscopy (NIRS) throughout the VOT. Vascular responsiveness within the microvasculature was evaluated by the re-oxygenation Slope 2 (Slope 2 StO2) and the area under the curve (StO2AUC) of (StO2) signal during re-oxygenation in the leg and arm.
Results
There was a significant interaction between training status and limb for the slope 2 StO2 (P < 0.01). The leg of the trained group showed a steeper slope 2 StO2 (1.35 ± 0.12% s−1) when compared to the slope 2 StO2 of the leg in their untrained counterparts (0.86 ± 0.09% s−1) (P < 0.05). There was a medium effect size of 0.58 for slope 2 StO2 on the arm and a large effect size of 1.21 for slope 2 StO2 on the leg. In addition, there was a small effect size of 0.24 for StO2AUC on the arm and a medium effect size of 0.64 for StO2AUC on the leg.
Conclusion
The present study suggests that the vascular adaptations induced by lower limb endurance exercise training are more prominent in the trained limb than in the untrained limb microvasculature.



Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- FDS:
-
Flexor digitorum superficialis
- FMD:
-
Flow-mediated dilation
- HbO2 :
-
Oxygenated hemoglobin
- HHb:
-
Deoxygenated hemoglobin
- NIRS:
-
Near-infrared spectroscopy
- Slope 2 StO2 :
-
Upslope of the StO2 signal over a 10 s period immediately following cuff release.
- StO2 :
-
Oxygen saturation
- StO2AUC :
-
Total area under the re-oxygenation curve, above the baseline value until the end of the 8 min post cuff release
- TA:
-
Tibialis anterior
- VO2max :
-
Maximal oxygen consumption
- VOT:
-
Vascular occlusion test
References
Bender SB, Laughlin MH (2015) Modulation of endothelial cell phenotype by physical activity: impact on obesity-related endothelial dysfunction. Am J Physiol Heart Circ Physiol 309(1):H1–H8
Charles M, Charifi N, Verney J, Pichot V, Feasson L, Costes F, Denis C (2006) Effect of endurance training on muscle microvascular filtration capacity and vascular bed morphometry in the elderly. Acta Physiol 187(3):399–406
DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR (2000) Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102(12):1351–1357
Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR (2001) Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol 534(1):287–295
Early KS, Stewart A, Johannsen N, Lavie CJ, Thomas JR, Welsch M (2017) The effects of exercise training on brachial artery flow-mediated dilation: a meta-analysis. J Cardiopulm Rehabilit Prev 37(2):77–89
Eiken O, Kölegård R (2004) Comparison of vascular distensibility in the upper and lower extremity. Acta Physiol 181(3):281–287
Florescu M, Stoicescu C, Magda S, Petcu I, Radu M, Palombo C, Cinteza M, Lichiardopol R, Vinereanu D (2010) “Supranormal” cardiac function in athletes related to better arterial and endothelial function. Echocardiography 27(6):659–667
Gokce N, Vita JA, Bader DS, Sherman DL, Hunter LM, Holbrook M, O’Malley C, Keaney JF, Balady GJ (2002) Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol 90(2):124–127
Grassi B, Quaresima V (2016) Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21(9):091313–091313
Green DJ, Maiorana A, O’driscoll G, Taylor R (2004) Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561(1):1–25
Green DJ, Rowley N, Spence A, Carter H, Whyte G, George K, Naylor LH, Cable NT, Dawson EA (2013) Why isn’t flow-mediated dilation enhanced in athletes? Med Sci Sports Exerc 45(1):75–82
Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH (2017) Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97(2):495–528
Haun CT, Kephart WC, Holland AM, Mobley CB, McCloskey AE, Shake JJ, Pascoe DD, Roberts MD, Martin JS (2016) Differential vascular reactivity responses acutely following ingestion of a nitrate rich red spinach extract. Eur J Appl Physiol 116(11):2267–2279. https://doi.org/10.1007/s00421-016-3478-8
Hughes WE, Ueda K, Casey DP (2016) Chronic endurance exercise training offsets the age-related attenuation in contraction-induced rapid vasodilation. J Appl Physiol 120(11):1335–1342
Joyner MJ, Casey DP (2015) Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95(2):549–601
Kim C, Choi HE, Jung H, Kang SH, Kim JH, Byun YS (2014) Impact of aerobic exercise training on endothelial function in acute coronary syndrome. Ann Rehabil Med 38(3):388–395
Kroese A (1977) Reactive hyperaemia in the calf of trained and untrained subjects: a study with strain gauge plethysmography. Scand J Clin Lab Investig 37(2):111–115
Lambertucci RH, Levada-Pires AC, Rossoni LV, Curi R, Pithon-Curi TC (2007) Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev 128(3):267–275
Laughlin MH, Newcomer SC, Bender SB (2008) Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104(3):588–600
Malkinson T Maximal aerobic power in endurance trained and sedentary men and women, 10–74 years of age. In: Science and technology for humanity (TIC-STH), 2009 IEEE Toronto International Conference (2009) IEEE, pp 18–21
Mattioni Maturana F, Peyrard A, Temesi J, Millet GY, Murias JM (2018) Faster \({\dot{V}}\)O2 kinetics after priming exercises of different duration but same fatigue. J Sports Sci 36(10):1095–1102
McLay KM, Fontana FY, Nederveen JP, Guida FF, Paterson DH, Pogliaghi S, Murias JM (2016a) Vascular responsiveness determined by near-infrared spectroscopy measures of oxygen saturation. Exp Physiol 101(1):34–40
McLay KM, Gilbertson JE, Pogliaghi S, Paterson DH, Murias JM (2016b) Vascular responsiveness measured by tissue oxygen saturation reperfusion slope is sensitive to different occlusion durations and training status. Exp Physiol 101(10):1309–1318. https://doi.org/10.1113/ep085843
McLay KM, Nederveen JP, Pogliaghi S, Paterson DH, Murias JM (2016c) Repeatability of vascular responsiveness measures derived from near-infrared spectroscopy. Physiol Rep 4(9):e12772
Montero D, Walther G, Diaz-Cañestro C, Pyke KE, Padilla J (2014) Microvascular dilator function in athletes: a systematic review and meta-analysis. Med Sci Sports Exerc 47:1485–1494
Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ (2001) Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88(2):145–151
Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN (2004) Different vasodilator responses of human arms and legs. J Physiol 556(3):1001–1011
Pauw KD, Roelands B, Cheung SS, De Geus B, Rietjens G, Meeusen R (2013) Guidelines to classify subject groups in sport-science research. Int J Sports Physiol Perform 8(2):111–122
Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ (2008) Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 295(1):R236–R242
Rizzoni D, Aalkjaer C, De Ciuceis C, Porteri E, Rossini C, Rosei CA, Sarkar A, Rosei EA (2011) How to assess microvascular structure in humans. High Blood Press Cardiovasc Prev 18(4):169–177
Soares RN, Murias JM (2018) Near-infrared spectroscopy assessment of microvasculature detects difference in lower limb vascular responsiveness in obese compared to lean individuals. Microvasc Res 118:31–35
Soares RN, McLay KM, George MA, Murias JM (2017a) Differences in oxidative metabolism modulation induced by ischemia/reperfusion between trained and untrained individuals assessed by NIRS. Physiol Rep 5(19):e13384
Soares RN, Reimer RA, Alenezi Z, Doyle-Baker PK, Murias JM (2017b) Near-infrared spectroscopy can detect differences in vascular responsiveness to a hyperglycaemic challenge in individuals with obesity compared to normal-weight individuals. Diabetes Vasc Dis Res 15:1479164117731481
Soares RN, Reimer RA, Murias JM (2017c) Changes in vascular responsiveness during a hyperglycemia challenge measured by near-infrared spectroscopy vascular occlusion test. Microvasc Res 111:67–71
Soares RN, Schneider A, Valle SC, Schenkel PC (2018) The influence of CYP1A2 genotype in the blood pressure response to caffeine ingestion is affected by physical activity status and caffeine consumption level. Vasc Pharmacol. https://doi.org/10.1016/j.vph.2018.03.002
Spence AL, Carter HH, Naylor LH, Green DJ (2013) A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise. Conduit artery adaptation in humans. J Physiol 591(5):1265–1275
Tanaka LY, Bechara LRG, dos Santos AM, Jordão CP, de Sousa LGO, Bartholomeu T, Ventura LI, Laurindo FRM, Ramires PR (2015) Exercise improves endothelial function: a local analysis of production of nitric oxide and reactive oxygen species. Nitric Oxide 45:7–14
Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ (2008) Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586(20):5003–5012
Walther G, Nottin S, Karpoff L, Pérez-Martin A, Dauzat M, Obert P (2008) Flow-mediated dilation and exercise-induced hyperaemia in highly trained athletes: comparison of the upper and lower limb vasculature. Acta Physiol 193(2):139–150
Acknowledgements
The first author acknowledge the financial support provided by CAPES (Brazil).
Author information
Authors and Affiliations
Contributions
RS analyzed the data, contribute to the data interpretation, and wrote the manuscript. MAG conducted the experiments. DNP contributed to the data interpretation and manuscript writing. JMM designed the research, contributed to the data interpretation and manuscript writing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Keith Phillip George.
Rights and permissions
About this article
Cite this article
Soares, R.N., George, M.A., Proctor, D.N. et al. Differences in vascular function between trained and untrained limbs assessed by near-infrared spectroscopy. Eur J Appl Physiol 118, 2241–2248 (2018). https://doi.org/10.1007/s00421-018-3955-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3955-3