Abstract
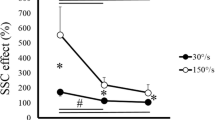
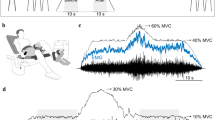
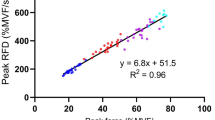
This study investigated the influence of different initial conditions on a subsequent fast (ballistic) isometric contraction of the ankle dorsiflexor muscles. Surface electromyograms (EMGs) of dorsiflexor and plantarflexor muscles were recorded during ballistic contractions performed without any pre-activation (BAL) and in ballistic contractions preceded by a sustained submaximal contraction (20% MVC) that was followed either by a rapid voluntary relaxation of the agonist muscle (VRBAL) or by a rapid antagonist (reversal) contraction (ARBAL). In the latter condition, three different antagonist torque levels were compared (25, 50 and 75% MVC). The results showed that the mean average rate of torque development was significantly (P < 0.001) greater for the ARBAL condition (968.5 ± 183.9% MVC/s) compared with the VRBAL (509.3 ± 78.7% MVC/s) and BAL (461.8 ± 79.9% MVC/s) conditions. Furthermore, the mean value recorded for VRBAL was significantly (P < 0.05) greater than for BAL condition. The faster increases in torque during the VRBAL and ARBAL conditions were associated with a greater agonist EMG activity. Compared with VRBAL, performance during the ARBAL condition was improved by a greater level of antagonist coactivation and, in some trials, by the presence of a silent EMG period between the end of the antagonist activation and the onset of the agonist ballistic contraction. Together, these results indicate that the initial conditions can have a substantial influence on the rate of torque development during ballistic contractions performed in isometric conditions.




Similar content being viewed by others
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93:1318–1326
Agostino R, Hallet M, Sanes JN (1992) Antagonist muscle inhibition before rapid voluntary movements of the human wrist. Electroencephalogr Clin Neurophysiol 85:190–196
Angel RW (1975) Myoelectric patterns associated with ballistic movement: effect of unexpected changes in load. J Hum Mov Stud 1:96–103
Aoki H, Tsukahara R, Yabe K (1989) Effects of pre-motion electromyographic silent period on dynamic exertion during a rapid ballistic movement in man. Eur J Appl Physiol 58:26–32
Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R (1988) Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med 16:113–122
Bawa P, Calancie B (1983) Repetitive doublets in human flexor carpi radialis muscle. J Physiol 339:123–132
Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD (1996) Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain 119:661–674
Carpentier A, Duchateau J, Hainaut K (1999) Load-dependent muscle strategy during plantar flexion in humans. J Electromyogr Kinesiol 9:1–11
Conrad B, Benecke R, Goehmann M (1983) Premovement silent period in fast movement initiation. Exp Brain Res 51:310–313
Cooke JD, Brown SH (1990) Movement-related phasic muscle activation. II. Generation and functional role of the triphasic pattern. J Neurophysiol 63:465–472
Corcos DM, Gottlieb GL, Agarwal GC (1989) Organizing principles for single-joint movements. II. A speed-sensitive strategy. J Neurophysiol 62:358–368
de Ruiter CJ, Kooistra RD, Paalman MI, de Haan A (2004) Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol 97:1693–1701
Desmedt JE, Godaux E (1977) Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol 264:673–693
Edman KA, Elzinga G, Noble MI (1978) Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol 281:139–155
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:143–169
Gruber M, Gollhofer A (2004) Impact of sensorimotor training on the rate of force development and neural activation. Eur J Appl Physiol 92:98–105
Hasan Z (1986) Optimized movement trajectories and joint stiffness in unperturbed, inertially loaded movements. Biol Cybern 53:373–382
Hortobagyi T, DeVita P (2000) Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J Electromyogr Kinesiol 10:117–126
Hufschmidt HJ, Hufschmidt T (1954) Antagonist inhibition as the earliest sign of a sensorimotor reaction. Nature 174:607
Ito M, Kawakami Y, Ichinose Y, Fukashiro S, Fukunaga T (1998) Nonisometric bevavior of fascicles during isometric contractions of a human muscle. J Appl Physiol 85:1230–1235
Lee JB, Matsumoto T, Othman T, Yamauchi M, Taimura A, Kaneda E, Ohwatari N, Kosaka M (1999) Coactivation of the flexor muscles as a synergist with the extensors during ballistic finger extension movement in trained kendo and karate athletes. Int J Sports Med 20:7–11
Lévénez M, Kotzamanidis C, Carpentier A, Duchateau J (2005) Spinal reflexes and coactivation of ankle muscles during a submaximal fatiguing contraction. J Appl Physiol 99:1182–1188
Marsden CD, Obeso JA, Rothwell JC (1983) The function of the antagonist muscle during fast limb movement in man. J Physiol 335:1–13
Moritani T (1993) Neuromuscular adaptations during the acquisition of muscle strength, power and motor tasks. J Biomech 26:95–107
Mortimer JA, Eisenberg P, Palmer SS (1987) Premovement silence in agonist muscles preceding maximum efforts. Exp Neurol 98:542–554
Mustard BE, Lee RG (1987) Relationship between EMG patterns and kinematic properties for flexion movements at the humen wrist. Exp Brain Res 66:247–256
Nishizono H, Kato M (1987) Inhibition of muscle activity prior to skilled voluntary movement. In: Jonsson B (ed) Biomechanics X-A. Human Kinetics, Champaign, IL, pp 455–458
Osternig LR, Hamill J, Lander JE, Robertson R (1986) Co-activation of sprinter and distance runner muscles in isokinetic exercise. Med Sci Sports Exerc 18:431–435
Palmer E, Cafarelli E, Ashby P (1994) The processing of human ballistic movements explored by stimulation over the cortex. J Physiol 481:509–520
Rassier DE, MacIntosh BR, Herzog W (1999) Length dependence of active force production in skeletal muscle. J Appl Physiol 86:1445–1457
Shibata M, Moritani T (1991) The mechanism of electromyographic silent periods preceding a ballistic voluntary plantar flexion. Ann Physiol Anthropol 10:211–218
Tanii K (1984) Occurence of a rhythmic slower wave in EMG prior to a rapid voluntary movement. Electroencephalogr Clin Neurophysiol 57:435–440
Tsukahara R, Aoki H, Yabe K, Mano T (1995) Effects of promotion silent period on single motor unit firing at initiation of a rapid contraction. Electroencephalogr Clin Neurophysiol 97:223–230
Van Cutsem M, Duchateau J (2005) Preceding muscle activity influences motor unit discharge and rate of torque development during ballistic contractions in humans. J Physiol 562:635–644
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513:295–305
Van Dieen JH, Cholewicki J, Radebold A (2003) Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine 28:834–841
Van Ingen Schenau GJ (1984) An alternative view of the concept of human utilization of elastic energy in human movement. Hum Mov Sci 3(4):301–336
Virji-Babul N, Cooke JD (1995) Influence of joint interactional effects on the coordination of planar two-joint arm movements. Exp Brain Res 103:451–459
Walter CB (1988) The influence of agonist premotor silence and the stretch-shortening cycle on contractile rate in active skeletal muscle. Eur J Appl Physiol 57:577–582
Walter CB (1989) Voluntary control of agonist premotor silence preceding limb movements of maximal effort. Percept Mot Skills 69:819–826
Wierzbicka MM, Wolf W, Staude G, Konstanzer A, Dengler R (1993) Inhibition of EMG activity in isometrically loaded agonist muscle preceding a rapid contraction. Electromyogr Clin Neurophysiol 33:271–278
Yabe K (1976) Premotion silent period in rapid voluntary movement. J Appl Physiol 41:470–473
Zehr EP, Sale DG (1994) Ballistic movement, muscle activation and neuromuscular adaptation. Can J Appl Physiol 19:363–378
Zehr EP, Sale DG, Dowling JJ (1997) Ballistic movement performance in karate athletes. Med Sci Sports Exerc 29:1366–1373
Acknowledgements
This study was supported by the Fonds National de la Recherche Scientifique (FNRS-FRS) of Belgium and the Conseil de la Recherche of the Université Libre de Bruxelles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Arnold de Haan.
Rights and permissions
About this article
Cite this article
Richartz, C., Lévénez, M., Boucart, J. et al. Initial conditions influence the characteristics of ballistic contractions in the ankle dorsiflexors. Eur J Appl Physiol 110, 805–814 (2010). https://doi.org/10.1007/s00421-010-1564-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1564-x