Abstract
Stressful exercise results in temporary immune depression. However, the impact of exercise on the immune responses via toll-like receptor (TLR) 7, which recognizes the common viral genomic feature, single-stranded RNA, remains unclear. To clarify the effect of stressful exercise on immune function in response to viral infection, we measured the changes in the plasma concentration of tumor necrosis factor (TNF)-α and interferon (IFN)-α, which are induced downstream from the TLR–ligand interaction, in exhaustive-exercised mice immediately after treatment with the imidazoquinoline R-848, which can bind to and activate TLR7. Both exhaustive-exercised (EX) and non-exercised (N-EX) male C3H/HeN mice were injected with R-848 (5 mg kg−1), and blood samples were collected. In addition, RAW264 cells, which are mouse macrophage cells, were cultured 30 min after epinephrine (10 μM) or norepinephrine (10 μM) treatments, and were then stimulated with R-848 (10 μg ml−1). In addition, the effect of propranolol (10 mg kg−1) as blockade of β-adrenergic receptors on R-848-induced TNF-α and IFN-α production in the exercised mice was examined. Both the TNF-α and IFN-α concentrations in the plasma of EX were significantly lower than those in the plasma of N-EX after R-848 injection (P < 0.05 and P < 0.01, respectively), although the R-848 treatment increased the plasma TNF-α and IFN-α concentrations in both groups (P < 0.01, respectively). The R-848-induced TNF-α production in RAW264 cells was significantly inhibited by epinephrine and norepinephrine pre-treatment, although IFN-α was not detected. The propranolol treatment completely inhibited exercise-induced TNF-α and IFN-α suppression in response to R-848 in the mice. These data suggest that EX induces a reduction in TNF-α and IFN-α production in response to R-848, and that these phenomena might be regulated by an exercise-induced elevation of the systemic catecholamines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nucleic acid in an RNA virus is usually single-stranded RNA (ssRNA). Among the infectious diseases caused by ssRNA viruses are influenza, norovirus infection, severe acute respiratory syndrome (SARS), and human immunodeficiency virus (HIV) infection (Mayo and Pringle 1998). Recent studies have identified the imidazoquinoline resiquimod (R-848) to be an activator of human and murine toll-like receptor (TLR) 7 (Hemmi et al. 2002) and human, but not murine, TLR8 (Jurk et al. 2002). R-848-induced immune activation is very similar to virus ssRNA-induced immune activation because both R-848 and viral ssRNA bind to TLR7 (Akira et al. 2006). In fact, pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α are induced by R-848 stimulation via nuclear factor-kappa (NF-κ) B activation, which lies downstream of the TLR–ligand interaction (Gibson et al. 2002). In addition, R-848 is a potent inducer of interferon (IFN)-α, an antiviral cytokine (Colonna et al. 2002), through interferon regulatory factor (IRF) 7 activation (Gibson et al. 2002; Heil et al. 2004; Karlsson et al. 2008).
In recent studies, it has been reported that stressful exercise might be associated with an increased susceptibility to viral infection by the herpes simplex virus (HSV) and influenza virus infections (Kohut et al. 2001; Murphy et al. 2008). In fact, stressful exercise has been shown to decrease the macrophage, neutrophil and natural killer cell functions (Davis et al. 1997; Ceddia and Woods 1999; Pyne et al. 2000; Kohut et al. 2001). In contrast, based on the findings of a bioassay sensitive to IFN-α, it has been suggested that there is a rise in the circulating concentration of IFN-α following acute exercise (Viti et al. 1985). However, the changes in IFN-α after pathogen stimulation during exercise and after exercise were not shown in that study. Accordingly, there is a possibility that changes in IFN-α in response to R-848 occurred with exhaustive exercise. We and other researchers have already shown that bacterial component-induced pro-inflammatory cytokine production is inhibited by acute exhaustive exercise (Bagby et al. 1994; Kato et al. 2006; Kitamura et al. 2007; Tanaka et al. 2010). Furthermore, Kitamura et al. (2007) suggested that exercise-induced catecholamines are responsible for exercise-induced suppression of pro-inflammatory cytokine production. However, it remains unclear whether the immune response to viral infection is also inhibited by intense exercise. In particular, the role of TLR7 on stressful exercise-induced increase in viral infection is not currently understood. It is also unclear whether exercise-induced catecholamines stimulate cytokine production in response to R-848. The purpose of the present study was to determine whether exhaustive exercise (EX) inhibits TNF-α and IFN-α production after R-848 injection in mice. Our hypothesis was that R-848-induced TNF-α and IFN-α production would be inhibited following exhaustive exercise.
Materials and methods
Animals and cell lines
Nine- or ten-week-old male C3H/HeN mice (n = 55) were purchased from Clea Japan (Osaka, Japan). These mice were housed individually in cages and were maintained on a 12:12 h light:dark cycle with free access to food and water. The experimental procedures and housing conditions were approved by the Animal Care and Use Committee of the Department of Health and Sports Science in Kawasaki University of Medical Welfare (HSS070006), and the experimental procedures also followed the guidelines set forth in the Care and Use of Animals in the Field of Physiological Sciences approved by the Council of the Physiological Society of Japan.
RAW 264 cells, a mouse-derived macrophage cell line, were obtained from the Cell Bank Riken Bioresource Center (Ibaraki, Japan). These cells were cultured in DMEM containing 10% FCS supplemented with 200 U ml−1 penicillin and 100 µg ml−1 streptomycin at 37°C, 5% CO2.
Drugs
R-848 was purchased from Calbiochem (Darmstadt, Germany). Epinephrine, norepinephrine and propranolol were obtained from Sigma (St. Louis, MO).
Experiment 1
The mice were randomly assigned to one of two groups. One group contained exhaustive-exercised mice, called EX (n = 11), and the other group contained non-exercised mice, called N-EX (n = 14). The EX mice were run on a treadmill to the point of exhaustion. The exercise protocol was as follows: the starting speed was 9 m min−1. This speed was increased 2 m min−1 every 3 min until it reached 17 m min−1. Thereafter, we continued to increase the speed by 1 m min−1 every 3 min until exhaustion. Exhaustion was defined as the point at which a mouse refused to run despite being given mild touches. Electric shock was not used during the treadmill run (Tanaka et al. 2010). In this study, the mean treadmill running time was 72 ± 5 min. N-EX mice were maintained in a sedentary condition for 60–70 min without access to food and water.
The animals were injected with R-848 (5 mg kg−1, i.v.). Immediately, each mouse was lightly anesthetized with the inhalant Isoflurane prior to i.v. injection into the orbital eye vessel. Blood samples were collected from the eye vessel before exercise, and at 1, 3, 6 and 24 h after R-848 injection in mice under Isoflurane anesthesia after exercise or rest. The samples were collected in EDTA-coated tubes and were immediately placed on ice. The plasma was isolated by centrifugation (2,000g, 20 min at 4°C) of whole blood and was stored at −40°C until assayed.
Experiment 2
RAW 264 cells (2 ×104 well−1) in 96-well plates were pre-incubated for 24 h and then were stimulated for 30 min with phosphate-buffered saline (PBS) as the vehicle, epinephrine (E, 10 µM) or norepinephrine (NE, 10 µM) and were then challenged with R-848 (10 µg ml−1) for 6 h. After the challenge, the supernatants were collected and then stored at −40°C until the analysis of TNF-α and IFN-α using ELISA.
Experiment 3
The mice were randomly assigned to one of three groups. One group was composed of exhausted exercised mice (EX, n = 10). Another group consisted of non-exercised mice (N-EX, n = 10). The third group was composed of exhausted exercised mice 30 min after treatment with propranolol (10 mg kg−1, i.p.) treatment, as a blocker of the β-adrenergic receptor (Prop+EX, n = 10). The exercise and resting protocols were the same as those described in Experiment 1. The mean times to exhaustion in EX and Prop+EX mice were 63 ± 3 and 58 ± 3 min, respectively (P = 0.305, not significant). Thereafter, all mice were injected with R-848 (5 mg kg−1, i.v.) immediately after exercise or rest. An hour after the R-848 injection, mouse blood samples were collected in EDTA-coated tubes and immediately placed on ice. The plasma was isolated by centrifugation (2,000g, 20 min at 4°C) of whole blood and stored at −40°C until analysis of TNF-α and IFN-α.
Assay
TNF-α (R&D Systems, Minneapolis, MN) and IFN-α (PBL, Piscataway, NJ) concentrations were analyzed using mouse ELISA kits. The minimum detectable concentrations of TNF-α and IFN-α were 5.1 and 12.5 pg ml−1, respectively. All sample determinations were measured in duplicate, and the mean values were then calculated. The coefficients of variation for the TNF-α and IFN-α assays were 4.03 and 5.08%, respectively.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical calculations were carried out with SPSS 15.0 for Windows software program. The data in Experiment 1 were analyzed using the two-way repeated-measures analysis of variance (ANOVA). Data in Experiment 2 were analyzed with two-way ANOVA. Data in Experiment 3 were analyzed with one-way ANOVA. Post-hoc Tukey’s or Bonferroni’s tests were performed. Data were considered to be significantly different when P values were less than 0.05.
Results
Effect of exhaustive exercise on TNF-α and IFN-α concentration in plasma in response to R-848
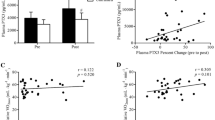
The changes in TNF-α concentration in plasma before and after R-848 injection in exhaustive-exercised and non-exercised mice are shown in Fig. 1. The plasma TNF-α concentrations varied as an effect of exercise; F(1,23) = 6.10, P < 0.05, time F(4,92) = 91.7, P < 0.01, and exercise × time interactions; F(4,92) = 4.99, P < 0.01. The plasma TNF-α concentration in N-EX mice was greatly increased at 1 h after R-848 injection (P < 0.01). In EX mice, however, the elevation of TNF-α concentration in plasma was not as high as that in the N-EX group at 1 and 3 h after R-848 injection (P < 0.05, respectively). This phenomenon, i.e., that R-848-induced TNF-α production is inhibited by prior exhaustive exercise, seems to be very similar to that of LPS-induced TNF-α production (Bagby et al. 1994; Kitamura et al. 2007; Tanaka et al. 2010). Therefore, intense exercise might inhibit R-848-induced pro-inflammatory cytokine production.
Effect of exhaustive exercise on plasma TNF-α concentrations in mice in response to R-848. Open circles represent the values in the non-exercised mice (N-EX, n = 14), and the closed circles represent these values in exercise mice (EX, n = 11). The plasma TNF-α concentration in the EX group was significantly lower than that in the N-EX group at 1 and 3 h after R-848 (5 mg kg−1, i.v.) injection (P < 0.05, respectively). *P < 0.05 vs. N-EX at each point and ## P < 0.01 vs. the value at Pre in each group
We examined the effect of exercise on R-848-induced anti-viral cytokine production. Figure 2 shows the changes in IFN-α concentrations in plasma before and after R-848 injection in exhaustive-exercised and non-exercised mice. The IFN-α concentration varied as an effect of the exercise; F(1,23) = 91.7, P < 0.01, time; F(4,92) = 85.5, P < 0.01, and those interactions; F(4,92) = 7.14, P < 0.01. Although the IFN-α concentration in the EX mice was markedly increased 1 and 3 h after the R-848 challenge, this level of concentration was significantly lower than that in the N-EX mice at 1 h after the R-848 challenge (P < 0.01, Fig. 2).
Effect of exhaustive exercise on plasma IFN-α concentrations in mice in response to R-848. The open circles represent those values in non-exercised mice (N-EX, n = 14), and the closed circles show these values in exercised mice (EX, n = 11). The increase in the IFN-α concentration in plasma was attenuated by exhaustive exercise 1 h after R-848 injection (P < 0.01). **P < 0.01 vs. N-EX at each point and ## P < 0.01 vs. the value at Pre in each group
Effect of epinephrine and norepinephrine pre-treatment on R-848-induced TNF-α and IFN-α production in macrophages in vitro
The changes in TNF-α and IFN-α production in response to R-848 after epinephrine (E) or norepinephrine (NE) treatments in RAW264 cells are shown in Fig. 3. The ANOVA results of the TNF-α production from RAW 264 cells indicate the R-848 and vehicle treatment; F(1,42) = 4,448, P < 0.01, the effect of the E and NE treatment; F(2,42) = 397, P < 0.01, and those interactions; F(2,42) = 394, P < 0.01. The R-848-induced TNF-α production from RAW264 cells was significantly inhibited by E and NE treatments (P < 0.01, respectively), and a significant difference was observed between TNF-α in E and in NE (P < 0.01).
Effect of epinephrine (E) and norepinephrine (NE) treatment on TNF-α and IFN-α production in response to R-848 in RAW264 cells. Cells (2 × 104 well−1) were incubated for 6 h with or without R-848 (10 µg ml−1) 30 min after treatment with E (10 µM) or NE (10 µM). The TNF-α production in both E- and NE-treated cells was significantly lower than that in vehicle (V)-treated cells after R-848 stimulation (P < 0.01, respectively). **P < 0.01 vs. V with R-848 and ## P < 0.01 vs. E with R-848. IFN-α was below the detection limits for all three treatments
Interestingly, the IFN-α production from RAW264 cells was not observed in any conditions. Although further experiments are needed for clarification, the result at least suggests that R-848 did not stimulate significant IFN-α production in macrophages. This result is consistent with previous studies using human monocytes and PBMC (Heil et al. 2004; Siren et al. 2005).
Effect of β-adrenergic receptor blockage on exercise-reduced TNF-α and IFN-α production in response to R-848
The changes in plasma TNF-α and IFN-α concentrations in response to R-848 after treatment with the β-adrenergic receptor antagonist propranolol in EX mice are shown in Fig. 4. Changes in the plasma TNF-α [F(2,27) = 10.9, P < 0.01], and IFN-α [F(2,27) = 8.56, P < 0.01] concentrations were observed. We found that exhaustive exercise reduced the R-848-induced plasma TNF-α levels seen in the N-EX mice (P < 0.01). However, the pretreatment of mice with the β-adrenergic receptor blocker propranolol almost completely reversed the exercise-induced suppression of plasma TNF-α in response to R-848 (EX vs. Prop+EX; < 0.01, Fig. 4a). In addition, the exercise-induced reduction of the plasma IFN-α concentratiPon was also completely inhibited by propranolol treatment (N-EX vs. EX; P < 0.01, and EX vs. Prop+EX; P < 0.01, Fig. 4b). These results suggest that EX-induced catecholamines reduce pro-inflammatory and type I cytokine productions in response to R-848.
Effect of the β-adrenagic receptor antagonist propranolol (Prop) on plasma TNF-α (a) and IFN-α (b) concentrations in response to R-848 in exhaustive-exercised mice. Mice were treated with Prop (10 mg kg−1, i.p.) 30 min before the exercise (Prop+EX). Plasma TNF-α and IFN-α concentrations in the EX group were significantly lower than those in the N-EX group 1 h after R-848 (5 mg kg−1, i.v.) injection (both **P < 0.01), but there were no significant differences between the plasma cytokine concentrations in the Prop+EX and the N-EX groups in response to R-848. ( ): n
Discussion
Prolonged strenuous exercise has been shown to decrease macrophage, neutrophil, and lymphocyte functions (Davis et al. 1997; Ceddia and Woods 1999; Pyne et al. 2000; Lancaster et al. 2005a). These studies provide evidence that exhaustive exercise induces immune depression, potentially weakening host defence against viral infections. Other studies have confirmed that prolonged or exhaustive exercise suppresses the antigen-specific cytokine response to upper respiratory infection (Kohut et al. 2001) and increases susceptibility to influenza infection (Murphy et al. 2008).
In this study, therefore, we examined whether exhaustive exercise may influence the plasma concentration of TNF-α and IFN-α in response to R-848 via TLR7. We thereafter examined the mechanisms of this effect. We found that exhaustive exercise reduces TNF-α and IFN-α production in response to R-848. In addition, R-848-induced TNF-α production in macrophages was inhibited by epinephrine and norepinephrine pre-treatment, although IFN-α was not detected. Moreover, we provided evidence that treatment with the β-adrenergic receptor blocker propranolol inhibits both exercise-induced TNF-α and IFN-α suppression in response to R-848.
R-848 binds to TLR7 or TLR8 (in the case of humans), and then activates NF-κB and IRF7, which induce the mRNA expression of pro-inflammatory cytokines (Gibson et al. 2002) and type I interferon (Heil et al. 2004; Schoenemeyer et al. 2005), respectively. It is clear that the signaling pathway downstream of TLR7 requires IRF7, which enhances the ability to bind to the IFN-α promoter after nuclear translocation. Therefore, only IFN-α is directly induced by viral infection, and IFN-α induction is a consequence of this initial IFN-α expression. During viral infections, the rapid production of IFN-α is required to prevent the spread of viruses within the host organism. In addition to its direct antiviral effects, IFN-α regulates both the innate and adaptive immunity. Accordingly, exhaustive exercise-induced IFN-α suppression may be one reason why prolonged strenuous exercise increases the risk for viral infection (Nieman 1994).
However, the question as to which stage (pathogen recognition, the mRNA expression of cytokine, mRNA translation, and/or post release neutralization) regulates immune depression after exercise remains unanswered, despite tremendous advances in our understanding of the role of TLRs in host defense and the specific signaling events that are initiated following TLR activation (Akira and Takeda 2004). Acute exercise has been reported to decrease the monocyte cell-surface expression of TLRs in humans (Gleeson et al. 2006; Lancaster et al. 2005; Stewart et al. 2005; Simpson et al. 2009). However, other studies found no effect of acute exercise on monocyte cell-surface TLR4 expression (McFarlin et al. 2004; Tanaka et al. 2010). The most likely explanation for the differences in these findings is related to the intensity/duration of the exercise stimulus and the form of the subject mammal. However, even if the expressions of TLR1, 2, 3 and 4, but not 9, were decreased by acute exercise (Gleeson et al. 2006; Lancaster et al. 2005b), the effect of the exercise on TLR7 expression still remains unclear. In addition, a recent study showed that EX-induced TNF-α suppression in response to LPS was observed despite no changes in the cell-surface expression of TLR4 and the TNF-α mRNA expression (Tanaka et al. 2010). Although this model of exercised animals with LPS injection might contribute to the study of immune function in response to bacterial infection, further studies are needed to clarify the detailed cellular and molecular mechanisms of immune functions in response to virus infection. Because it is well known that both LPS/TLR4 and R-848/TLR7 recognition induce NF-κB activation, and thereafter stimulate TNF-α mRNA expression (Akira et al. 2006), one possibility is that exhaustive exercise-induced TNF-α suppression in response to R-848 can also be regulated at the post-transcriptional stage of TNF-α in response to R-848.
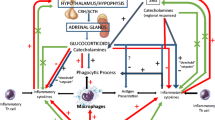
The differences in the concentrations of epinephrine and norepinephrine between exercised and non-exercised animals are considerable. Exhaustive exercise increases plasma epinephrine (3.0–3.7-fold) and norepinephrine (4.2–10.4-fold) in rodents (Kitamura et al. 2007; Krüger et al. 2008). These catecholamines bind to α- and β-adrenergic receptors on the external surface of the cell membranes. The hormone–receptor complexes interact with G proteins and activate adenylate cyclase which converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). cAMP activates protein kinase A, which subsequently phosphorylates certain intracellular proteins, thereby altering their activity (Tanaka et al. 2002; Collado-Hidalgo et al. 2006). Catecholamines have been shown to suppress TNF-α production in response to LPS via a β-adrenergic receptor (Elenkov and Chrousos 2002; Kitamura et al. 2007). In peripheral blood leukocytes and plasmacytoid dendritic cells (pDCs), the induction of interferon antiviral activity by double-stranded RNA (poly I:C) or CpG DNA was substantially inhibited by catecholamines (Collado-Hidalgo et al. 2006). In the present study, we found that epinephrine could completely inhibit and norepinephrine could partially, but significantly, inhibit the R-848-induced TNF-α production in RAW264 cells. Furthermore, the β-adrenergic receptor antagonist propranolol greatly attenuated exercise-induced suppression of TNF-α and IFN-α in response to R-848 in mice. Our data for the first time demonstrate the independent role of catecholamines in suppressing cytokine production in response to R-848 after exercise. It is known that catecholamines reduce macrophage production of pro-inflammatory cytokines such as TNF-α (Zinyama et al. 2001) and type I interferon such as IFN-α/β (Collado-Hidalgo et al. 2006). We hypothesized that catecholamines contribute to the exhaustive exercise-induced reduction in TNF-α and IFN-α levels following R-848. In an in vitro experiment, following R-848 treatment TNF-α production was significantly inhibited by pre-treatment with epinephrine and norepinephrine. It has been reported that β-adrenergic agonists suppress, and α-adrenergic agonists augment LPS-stimulated TNF-α production and its gene expression (Ignatowski et al. 1996). Norepinephrine has a high affinity for both α- and β-adrenergic receptors, whereas epinephrine has a high affinity for β-adrenergic receptors alone (Woods 2000). Therefore, the β-adrenergic receptors, which have an affinity for both epinephrine and norepinephrine, may modulate the effects of catecholamines on the exercise-induced changes in TNF-α in response to R-848.
In this study, however, R-848 did not stimulate any significant IFN-α production in macrophages in vitro. Recent studies have reported that R-848 could not induce IRF7 and IFN-α production in human macrophages (Heil et al. 2004; Siren et al. 2005). It remains to be determined which specific cell type is responsible for the increase in plasma IFN-α in response to R-848. Ito et al. (2002) previously reported that R-848 had the capacity to induce IFN-α production from pDCs, but not from CD11c+ myeloid DCs, in human peripheral blood. It is noteworthy that the pDCs constitutively express both β-adrenergic receptors (Goyarts et al. 2008) and TLR7 (Ito et al. 2002). Although it has been reported that exercise induced IL-12 production of DCs in animals (Chiang et al. 2007) and that acute exercise induced a rise in circulating DC counts in humans (Ho et al. 2001), unfortunately, it is not yet known whether the IFN-α production in DCs is induced by exhaustive exercise. At least, we could conclude that exercise-induced catecholamines acting through β-adrenergic receptors can significantly blunt TNF-α and IFN-α productions in response to R-848.
Conclusions
In summary, the present study was undertaken to determine whether exhaustive exercise inhibits TNF-α and IFN-α production after R-848 injection in mice. Both TNF-α and IFN-α concentrations in exercised mice were significantly lower than those in non-exercised mice. Furthermore, in an in vitro experiment, prior epinephrine treatment strongly inhibited R-848-induced TNF-α production in macrophages. Finally, the exercise-reduced TNF-α and IFN-α production in response to R-848 is completely inhibited by the blockade of β-adrenergic receptors. These results suggest that exhaustive exercise-induced catecholamines reduce cytokine production in response to R-848 via TLR7.
References
Akira S, Takeda K (2004) Functions of toll-like receptors: lessons from KO mice. C R Biol 327:581–589
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801. doi:10.1016/j.cell.2006.02.015
Bagby GJ, Sawaya DE, Crouch LD, Shepherd RE (1994) Prior exercise suppresses the plasma tumor necrosis factor response to bacterial lipopolysaccharide. J Appl Physiol 77:1542–1547
Ceddia MA, Woods JA (1999) Exercise suppresses macrophage antigen presentation. J Appl Physiol 87:2253–2258
Chiang LM, Chen YJ, Chiang J, Lai LY, Chen YY, Liao HF (2007) Modulation of dendritic cells by endurance training. Int J Sports Med 28:798–803. doi:10.1055/s-2007-964914
Collado-Hidalgo A, Sung C, Cole S (2006) Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav Immun 20:552–563. doi:10.1016/j.bbi.2006.01.005
Colonna M, Krug A, Cella M (2002) Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol 14:373–379. doi:10.1016/S0952-7915(02)00349-7
Davis JM, Kohut ML, Colbert LH, Jackson DA, Ghaffar A, Mayer EP (1997) Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol 83:1461–1466
Elenkov IJ, Chrousos GP (2002) Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann NY Acad Sci 966:290–303
Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP (2002) Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol 218:74–86. doi:10.1016/S0008-8749(02)00517-8
Gleeson M, McFarlin B, Flynn M (2006) Exercise and toll-like receptors. Exerc Immunol Rev 12:34–53
Goyarts E, Matsui M, Mammone T, Bender AM, Wagner JA, Maes D, Granstein RD (2008) Norepinephrine modulates human dendritic cell activation by altering cytokine release. Exp Dermatol 17:188–196. doi:10.1111/j.1600-0625.2007.00677.x
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004) Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526–1529. doi:10.1126/science.1093620
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3:196–200. doi:10.1038/ni758
Ho CS, López JA, Vuckovic S, Pyke CM, Hockey RL, Hart DN (2001) Surgical and physical stress increases circulating blood dendritic cell counts independently of monocyte counts. Blood 98:140–145
Ignatowski TA, Gallant S, Spengler RN (1996) Temporal regulation by adrenergic receptor stimulation of macrophage (M Φ)-derived tumor necrosis factor (TNF) production post-LPS challenge. J Neuroimmunol 65:107–117
Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S (2002) Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med 195:1507–1512. doi:10.1084/jem.20020207
Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S (2002) Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol 3:499. doi:10.1038/ni0602-499
Karlsson A, Jägervall K, Utkovic H, Karlsson L, Rehnström E, Fredin MF, Gillberg PG, Jansson L, Michaëlsson E, Melgar S (2008) Intra-colonic administration of the TLR7 agonist R-848 induces an acute local and systemic inflammation in mice. Biochem Biophys Res Commun 367:242–248. doi:10.1016/j.bbrc.2007.12.046
Kato T, Kaneko S, Kimizuka R, Okuda K (2006) Periodontopathic bacterial endotoxin- induced tumor necrosis factor alpha production was inhibited by exercise in mice. FEMS Immunol Med Microbiol 47:262–266. doi:10.1111/j.1574-695X.2006.00075.x
Kitamura H, Shiva D, Woods JA, Yano H (2007) Beta-adrenergic receptor blockade attenuates the exercise-induced suppression of TNF-alpha in response to LPS in rats. NeuroImmunoModulation 14:91–96. doi:10.1159/000107424
Kohut ML, Boehm GW, Moynihan JA (2001) Prolonged exercise suppresses antigen-specific cytokine response to upper respiratory infection. J Appl Physiol 90:678–684
Krüger K, Lechtermann A, Fobker M, Völker K, Mooren FC (2008) Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun 22:324–338. doi:10.1016/j.bbi2007.08.008
Lancaster GI, Khan Q, Drysdale PT, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M (2005a) Effect of prolonged strenuous exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol 98:565–571
Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M (2005b) The physiological regulation of toll-like receptor expression and function in humans. J Physiol 563:945–955. doi:10.1113/jphysiol.2004.081224
Mayo MA, Pringle CR (1998) Virus taxonomy–1997. J Gen Virol 79:649–657
McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL (2004) TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc 36:1876–1883
Murphy EA, Davis JM, Carmichael MD, Gangemi JD, Ghaffar A, Mayer EP (2008) Exercise stress increases susceptibility to influenza infection. Brain Behav Immun 22:1152–1155. doi:10.1016/j.bbi.2008.06.004
Nieman DC (1994) Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc 26:128–139
Pyne DB, Gleeson M, McDonald WA, Clancy RL, Perry C Jr, Fricker PA (2000) Neutrophil oxidative activity is differentially affected by exercise intensity and type. Int J Sports Med 21:S51–S60
Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT (2005) The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem 280:17005–17012. doi:10.1074/jbc.M412584200
Simpson RJ, McFarlin BK, McSporran C, Spielmann G, ó Hartaigh B, Guy K (2009) Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun 23:232–239. doi:10.1016/j.bbi.2008.09.013
Siren J, Pirhonen J, Julkunen I, Matikainen S (2005) IFN-α regulates TLR-dependent gene expression of IFN-α, IFN-β, IL-28, and IL-29. J Immunol 174:1932–1937
Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E (2005) Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun 19:389–397. doi:10.1016/j.bbi.2005.04.003
Tanaka KF, Kashima H, Suzuki H, Ono K, Sawada M (2002) Existence of functional beta1- and beta2-adrenergic receptors on microglia. J Neurosci Res 70:232–237. doi:10.1002/jnr.10399
Tanaka Y, Kawanishi N, Shiva D, Tsutsumi N, Uchida M, Kitamura H, Kato Y, Yano H (2010) Exhaustive exercise reduces tumor necrosis factor-alpha production in response to lipopolysaccharide in mice. NeuroImmunoModulation 17:279–286. doi:10.1159/000290044
Viti A, Muscettola M, Paulesu L, Bocci V, Almi A (1985) Effect of exercise on plasma interferon levels. J Appl Physiol 59:426–428
Woods JA (2000) Exercise and neuroendocrine modulation of macrophage function. Int J Sports Med 21:S24–S30
Zinyama RB, Bancroft GJ, Sigola LB (2001) Adrenaline suppression of the macrophage nitric oxide response to lipopolysaccharide is associated with differential regulation of tumor necrosis factor-alpha and interleukin-10. Immunology 104:439–446
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C-21500700) from the Japan Society for Promotion of Science (JSPS), and the Interdepartmental Research Fund of Kawasaki University of Medical Welfare (to H. Yano).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William Kraemer.
Rights and permissions
About this article
Cite this article
Yano, H., Uchida, M., Nakai, R. et al. Exhaustive exercise reduces TNF-α and IFN-α production in response to R-848 via toll-like receptor 7 in mice. Eur J Appl Physiol 110, 797–803 (2010). https://doi.org/10.1007/s00421-010-1560-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1560-1