Abstract
Skeletal muscle atrophy during sepsis, immobilization, and chronic diseases is characterized by an increase in expression and activity of the muscle-specific ubiquitin 3 ligases atrogin-1 and MuRF-1. The classical renin–angiotensin system (RAS), by high level of circulating angiotensin II (AngII) is directly involved in skeletal muscle wasting associated with cardiac and renal failure. Ang (1–7), a peptide belonging to the non-classical RAS system, produces effects that are opposite to AngII. The actions of Ang (1–7) are mediated by its binding and signalling through the Mas receptor. Our purpose is to assess the effects of atrophic stimuli AngII, lipopolysaccharide (LPS), and immobilization on the expression of the Mas receptor in skeletal muscle. For that we used gastrocnemius and tibialis anterior muscles of C57BL10 mice treated with AngII, LPS or subjected to unilateral hindlimb immobilization by casting. In addition, we used C2C12 myotubes incubated with AngII or LPS. We evaluated Mas expression by quantitative real-time PCR, Western blot immunohistochemical analysis. Skeletal muscle atrophy was corroborated by the expression of atrogin-1 and MuRF-1 and the fibre diameter. Our results show that Mas receptor expression was increased by AngII or LPS in vitro and in vivo, and upregulated by immobilization. The increase of the Mas expression was concomitantly with the upregulation of atrogin-1 and MuRF-1 and the reduction of the fibre diameter. These results from studies in vitro and in vivo demonstrate for the first time that the Mas receptor is increased under atrophic stimulus and suggest that the non-classical RAS system could have an important role in muscle wasting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle wasting or atrophy is characterized by a weakness generated by the loss of muscle mass concomitantly with a decrease of the ability to produce strength. One of the main mechanisms involved in skeletal muscle atrophy is an increase in protein degradation dependent on the activation of the ubiquitin–proteasome system (UPS) (Rezk et al. 2012; Sanders et al. 2005). The muscle-specific E3 ubiquitin ligases atrogin-1 and MuRF-1 belong to the UPS and are involved from the initial stages of skeletal muscle atrophy (Foletta et al. 2011; Glass 2003; Gomes et al. 2001; Gumucio and Mendias 2013); thus, they are upregulated in muscle atrophy caused by different situations (Bodine et al. 2001; Gomes et al. 2001; Semprun-Prieto et al. 2011). The causes of skeletal muscle atrophy are diverse and include sepsis, immobilization, and chronic diseases. A key factor involved in some chronic diseases, such as cardiac, renal, and pulmonary failure, is the participation of the renin–angiotensin system (RAS) by a high level of circulating angiotensin II (AngII) (Agarwal 2003; Agarwal et al. 2004; Antoniu 2008; Brecher 1996; Mancini and Khalil 2005).
The RAS is known for its systemic effects as a regulator of blood pressure. In addition, the RAS also affects other local functions that include the normal and pathological processes of several target tissues. The RAS is composed of two axes with contrary functions: the classical RAS includes AngII, its AT1 and AT2 receptors, and the angiotensin-converting enzyme (ACE), the enzyme forming AngII; whereas the non-classical RAS comprises Ang (1–7), its receptor Mas, and ACE-2, the enzyme that produces Ang (1–7). There is evidence that a balance between these axes is normally maintained, but is disturbed in pathological conditions (Ferreira et al. 2012a; Fyhrquist and Saijonmaa 2008; Iwai and Horiuchi 2009; Lubel et al. 2008).
Ang (1–7) promotes several biological actions, including the inhibition of cell proliferation, vasodilation, and antihypertensive effects (Benter et al. 1995; Ferrario et al. 2005; Iwata et al. 2005; Marangoni et al. 2006; Tallant et al. 2005). To date, one of the main effects of Ang (1–7) is the prevention or decrease of fibrosis (Grobe et al. 2006; Iwata et al. 2005). The effects of Ang (1–7) are mediated by the G-protein-coupled transmembrane receptor Mas (Chappell 2007; Ferreira et al. 2012b; Santos et al. 2003). In mammals, the gene is expressed predominantly in testis and in distinct areas of the forebrain, such as the hippocampus and amygdala, and less strongly but detectable in kidney, heart, lung, liver, spleen, tongue, and skeletal muscle (Metzger et al. 1995; Munoz et al. 2010; Villar and Pedersen 1994). Interestingly, Mas expression has been described to be modulated by pathological conditions in cardiac muscle and depending on the stimulus can be observed as an increase or a diminution (Chen et al. 2013; Dias-Peixoto et al. 2012).
Several studies have indirectly demonstrated the expression of Mas in skeletal muscle (Acuna et al. 2014; Morales et al. 2014; Prasannarong et al. 2012), although recently its level in rat soleus muscle was reported (Echeverria-Rodriguez et al. 2013). Other studies have demonstrated the expression of Mas in skeletal muscle through its participation in the beneficial effects of its ligand Ang (1–7) (Munoz et al. 2010, 2012). In this context, the participation of Mas was reported in the Ang (1–7)-dependent regulation of the insulin-signalling pathway and in glucose transport in skeletal muscle (Munoz et al. 2010, 2012; Prasannarong et al. 2012). Our group recently demonstrated that Ang (1–7), through Mas, counteracts the effects and signalling dependent on AngII in skeletal muscle tissue and cells (Morales et al. 2014), which is according to the report by Acuna et al. (2014) showing the critical role of Mas in controlling skeletal muscle damage and fibrosis by Ang (1–7) in Duchenne muscular dystrophy.
Despite the participation of the classical RAS axis in skeletal muscle atrophy, the participation and expression of Mas in muscle wasting has not been described. The purpose of this study was to assess the expression of Mas in skeletal muscle atrophy caused by several stimuli, such as AngII, lipopolysaccharide (LPS), and immobilization. Our results demonstrate that AngII and LPS increase mRNA and protein levels of Mas in myotubes in vitro and gastrocnemius and tibialis anterior muscles. In addition, in a model of unilateral immobilization by casting, Mas expression is upregulated. This is the first report that demonstrates a regulation of Mas expression under atrophic conditions.
Materials and methods
Animals
We used the C57BL/10J (12-week old) strain of mice (male). Animals were kept at room temperature with a 24 h night–day cycle, water available ad libitum, and paired feeding with pellets. The animals were randomized and separated into experimental groups (four to six animals/group), and three independent experiments were performed. At the end of each experiment, the animals were euthanized under anaesthesia and the gastrocnemius and tibialis anterior muscles were dissected, removed, and rapidly frozen and stored at −80 °C until processing (Morales et al. 2013a). All protocols were conducted in strict accordance and with the formal approval of the Animal Ethics Committee at the Universidad Andrés Bello.
Treatments
AngII (1 μg/kg/min) was osmotically infused through micropumps (Alzet-Durect, USA) implanted under ketamine/xylazine anaesthesia in the dorsal area of the animal for 1 or 14 days (Acuna et al. 2014). LPS from E. coli (Sigma, USA; 1 mg/kg) was i.p injected for 18 h or 14 days. Unilateral immobilization was performed in the lower hindlimb for 14 days using a 3M™ Scotchcast™ Soft Cast Casting Tape (Madaro et al. 2008).
Cell cultures
The skeletal muscle cell line C2C12 (American Type Culture Collection) was grown and differentiated until day 5, as described previously (Cabello-Verrugio et al. 2011; Painemal et al. 2013). The myotubes were incubated with 500 nM AngII (Sigma, USA) during 1 or 3 h for quantitative real-time PCR (RT-qPCR) or 24 h for Western blot. Myotubes were treated with 500 ng/ml of LPS (Sigma, USA) during 1–5 h for RT-qPCR or 48 h for Western blot. For treatment with the AngII receptor type 1 (AT-1) and type 2 (AT-2) blockers, the myotubes were pre-incubated for 1 h with losartan (10 μM) or PD-123319 (10 μM) (both from Tocris Bioscience, USA), respectively, and subsequently incubated with AngII. A similar procedure was followed for the inhibitor of the TLR-4 receptor CLI-095 (5 μM) (Invivogen, USA) prior to the incubation with LPS.
RNA isolation, reverse transcription and quantitative real-time PCR (RT-qPCR)
Total RNA was isolated from the gastrocnemius muscles, using TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. The total RNA (1 μg) was reverse transcribed to cDNA using random hexamers and Superscript II reverse transcriptase (Invitrogen). TaqMan RT-qPCRs were performed in triplicate using an Eco Real-Time PCR System (Illumina, USA) with pre-designed primer sets for mouse Mas, atrogin-1, MuRF-1, and the housekeeping gene beta actin (TaqMan Assays-on-Demand; Applied Biosystems, USA). mRNA expression was quantified using the comparative ΔCt method (2−ΔΔCT) with beta actin as the reference gene. The mRNA levels were expressed relative to the mean expression in the control condition (Morales et al. 2012).
Immunoblot analysis
For the skeletal muscle extracts, the gastrocnemius muscles were homogenized in Tris–EDTA buffer with a cocktail of protease inhibitors and 1 mM PMSF. Proteins were subjected to SDS-PAGE, transferred onto PDVF membranes (Millipore, USA), and probed with goat anti-Mas (1:500) (sc-54848, Santa Cruz Biotechnology, USA) and mouse anti-tubulin (1:5,000) (sc-5286, Santa Cruz Biotechnology, USA). All immunoreactions were visualized by enhanced chemiluminescence (Thermo Scientific, USA).
Immunohistochemical analysis
For immunohistochemistry, fresh-frozen gastrocnemius and tibialis anterior muscles cryosections (7 μm) were fixed in acetone, incubated for 1 h with anti-Mas (1:50) (sc-54848, Santa Cruz Biotechnology, USA) in 5 % goat serum in PBS, and blocked for 15 min in methanol–H2O2 3 %. After 1 h incubation with rabbit anti-goat-HRP (Thermo Scientific, USA) followed by 30 min incubation with Envision™ Dual Link System-HRP (Dako, USA), enzyme activity was detected by the use of a 3′,3′-diaminobenzidine tetrahydrochloride liquid system (Dako). Nuclei were stained with haematoxylin (Morales et al. 2011).
Skeletal muscle histology
Fresh-frozen gastrocnemius and tibialis anterior muscles were sectioned, and cryosections (7 µm) were placed on glass slides. Haematoxylin and eosin staining was performed according to standard procedures (Cabello-Verrugio et al. 2012b).
Fibre diameter determination and quantification
Fibre diameter was detected by the analysis of the H&E staining. Briefly, the sections stained with H&E were viewed and photographed on the Motic BA310 microscope (Motic, Hong Kong). Fibre sizes were determined using the ImageJ software (NIH, USA) on five randomly captured images of gastrocnemius and tibialis anterior muscles of each experimental condition (in a blind fashion). Fibres were manually selected and the minimal Feret’s diameter of each fibre was quantified by the ImageJ software (Morales et al. 2013b).
Statistics
Statistical analysis was evaluated using the Student’s t test or the one-way analysis of variance (ANOVA) with a post hoc Bonferroni multiple-comparison test (Sigma Stat software). A difference was considered statistically significant at a P value <0.05.
Results
Angiotensin II increases the expression of the Mas receptor in skeletal muscle in vitro and in vivo
We evaluated the effects of AngII on the mRNA levels of Mas receptor in C2C12 myotubes. For this, the cells were incubated with AngII for several times for RNA extraction. Figure 1a shows an increment in Mas expression between 1 and 9 h after AngII incubation, reaching a maximum effect of 3.5-fold at 3 h and a return to basal levels at 14 h. This increase was dependent on the AT-1 receptor (Fig. S1), which is demonstrated by the complete inhibition of Mas upregulation induced by AngII in the presence of losartan (an AT-1 receptor blocker) but not with PD123319 (an AT-2 receptor blocker). At the protein level, AngII induces the expression of Mas receptor in the myotubes twofold (Fig. 1b, c). In addition, an increase in atrogin-1 (Fig. 1d) and MuRF-1 (Fig. 1e) was detected, which verified the atrophic effect of AngII.
AngII increases the expression of the Mas receptor in C2C12 myotubes. a C2C12 myotubes from day 5 were incubated with AngII (500 nM) for the times indicated in the figure. At the end of this treatment, the mRNA levels of Mas were determined by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control cells. The values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control cells). b Myotubes were incubated with AngII (500 nM) for 24 h. At the end of the treatment, the levels of Mas were detected by Western blot analysis. The levels of tubulin are shown as the loading control. Molecular weights are indicated in kilodaltons (kDa). c The values of the quantification are expressed as the fold of induction relative to the control cells. The expressions of atrogin-1 (d) and MuRF-1 (e) were detected to corroborate the atrophic effect of AngII after 1- and 3-h treatment, respectively. The value corresponds to the mean ± SD of three independent experiments (*P < 0.05 relative to the control cells treated with the vehicle)
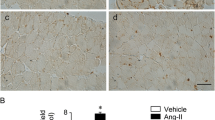
To evaluate the effect of AngII on the Mas receptor in vivo, we administrated AngII to mice through osmotic pumps. Figure 2a shows a twofold increase of Mas mRNA levels in gastrocnemius muscles of mice treated with AngII relative to control mice. This result is according to the twofold increase induced by AngII in the protein levels of Mas (Fig. 2b, c). The increase of Mas levels induced by AngII was corroborated in gastrocnemius muscle sections by immunohistochemistry (Fig. 2d) which was also observed in tibialis anterior (Fig. S2a). Atrophy induced by AngII was determined by a decrease in fibre diameter in gastrocnemius (Fig. 2e, f) and in tibialis anterior (Fig. S2b), and also for an increase in the expression of atrogin-1 (Fig. 2g) and MuRF-1 (Fig. 2h). Together, these results suggest that AngII increases Mas expression concomitant with the induction of atrophy in vitro and in vivo.
Systemic administration of AngII in mice increases the expression of the Mas receptor in skeletal muscle. C57BL10 mice were systemically treated with the vehicle (control; Ctrl) or AngII for 1 or 14 days, as described in “Materials and methods”. At the end of the treatment, the muscle gastrocnemius was removed and further processed. a mRNA levels of Mas were determined after 1 day of AngII administration by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control cells). b Protein levels of Mas receptor determined after 14 days of AngII administration by Western blot analysis. The levels of tubulin are shown as the loading control. Molecular weights are indicated in kilodaltons (kDa). c Quantitative analysis of the experiments shown in (b). Values are expressed as the fold of induction relative to the control cells. d Cryosections of the gastrocnemius obtained after 14 days of AngII administration were used to immunodetect Mas through immunohistochemical analysis. Nuclei were labelled with haematoxylin. The bar corresponds to 50 μm. The images are representative of three independent experiments, using four mice for each experimental condition. Upper ×10 magnification; lower ×40 magnification. e Histological analysis of gastrocnemius from control and AngII-treated mice for 14 days through haematoxylin and eosin stain in cryosections. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. Upper ×10 magnification; lower ×40 magnification. f Quantitative analysis of the fibre diameter from three independent experiments using three mice for each experimental condition. The values are expressed as the percentage of the total fibres quantified (*P < 0.05 relative to the control). g Atrogin-1 and h MuRF-1 mRNA levels were determined by RT-qPCR at 1 day after treatment with AngII, using β-actin as the reference gene. Levels were expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control)
Expression of the Mas receptor is increased by lipopolysaccharide in myotubes and skeletal muscle
The effect of LPS on the mRNA levels of the Mas receptor was evaluated by RT-qPCR in C2C12 myotubes. Figure 3a shows a 2.9- and 2.2-fold transient increase in Mas expression at 3 and 4 h, respectively, after LPS incubation, reaching basal levels at 5 h. This increase was dependent on the TLR-4 receptor for LPS since complete inhibition of LPS-induced Mas expression was observed in the presence of CLI-095 (a blocker of TLR-4) (Fig. S3). In addition, LPS also increased Mas protein levels (Fig. 3b). This augmented level was twice that of the control myotubes (Fig. 3c) and was concomitant with the increase observed for atrogin-1 (Fig. 3d) and MuRF-1 (Fig. 3e). We then evaluated the effect of LPS injected i.p. in mice on the expression of Mas in gastrocnemius muscles. Mas mRNA levels (Fig. 4a) and protein levels (Fig. 4b, c) in gastrocnemius muscles of mice treated with LPS increased twofold relative to control mice. In addition, we observed a strong increase in Mas levels in the plasma membrane of fibres evaluated in muscle sections by immunohistochemical analysis (Fig. 4d). Similar results were obtained for Mas levels in muscle TA (Fig. S4a). The atrophy induced by LPS was corroborated by a decrease in fibre diameter in gastrocnemius (Fig. 4e, f) and tibialis anterior (Fig. S4b) and also for an increase in the expression of atrogin-1 (Fig. 4g) and MuRF-1 (Fig. 4h). Thus, our results obtained in vitro and in vivo suggest that LPS induces an increase of the expression of Mas receptor together with typical parameters of skeletal muscle atrophy.
Expression of Mas is upregulated by LPS in C2C12 myotubes. a C2C12 myotubes from day 5 were incubated with LPS (500 ng/ml) for the times indicated in the figure. At the end of this treatment, the mRNA levels of Mas were determined by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control cells). b Myotubes were incubated with LPS (500 ng/ml) for 48 h. At the end of the treatment, the levels of Mas were detected by Western blot analysis. The levels of tubulin are shown as the loading control. c Values of the quantification are expressed as the fold of induction relative to the control cells. d Atrogin-1 and e MuRF-1 were detected to corroborate the atrophic effect of LPS. The value corresponds to the mean ± SD of three independent experiments (*P < 0.05 relative to the control cells treated with the vehicle)
Mas receptor levels are augmented in LPS-induced skeletal muscle atrophy. C57BL10 mice were intraperitoneally injected with LPS (1 mg/kg) for 18 h or 14 days, as described in “Materials and methods”. At the end of the treatment, muscle gastrocnemius was removed and processed. a mRNA levels of Mas were determined after 18 h of LPS treatment by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control cells). b Mas receptor protein levels were evaluated by Western blot in gastrocnemius from control and LPS-treated mice for 14 days. Tubulin is shown as the loading control. Molecular weights are indicated in kilodaltons (kDa). c Quantitative analysis of the experiments shown in (b). The values are expressed as the fold of induction relative to the control cells. d Cryosections of the gastrocnemius obtained after 14 day of AngII administration were used to immunodetect Mas through immunohistochemical analysis. Nuclei were labelled with haematoxylin. The bar corresponds to 50 μm. The images are representative of three independent experiments, using four mice for each experimental condition. Upper ×10 magnification; lower ×40 magnification. e Histological analysis of gastrocnemius from control and AngII-treated mice for 14 days through haematoxylin and eosin stain in cryosections. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. Upper ×10 magnification; lower ×40 magnification. f Quantitative analysis of the fibre diameter from three independent experiments using three mice for each experimental condition. The values are expressed as the percentage of the total fibres quantified (*P < 0.05 relative to the control). g Atrogin-1 and h MuRF-1 mRNA levels were determined by RT-qPCR at 18 h after treatment with LPS, using β-actin as the reference gene. The levels were expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control)
Expression of the Mas receptor is increased during immobilization-induced skeletal muscle atrophy
To evaluate the effect of immobilization on the expression of Mas, we detected its mRNA levels by RT-qPCR technique in gastrocnemius muscles. Mas mRNA levels in immobilized gastrocnemius muscles increased sixfold relative to the contralateral non-immobilized muscle (Fig. 5a) and protein levels increased 2.3-fold (Fig. 5b, c). In addition, we observed a strong increase in immunodetection of Mas levels in the plasma membrane of muscle fibres (Fig. 5d). Similar results were observed for Mas levels in immobilized tibialis anterior muscle (Fig. S5a). The atrophy induced by immobilization was corroborated by a decrease in fibre diameter in gastrocnemius (Fig. 5e, f) and tibialis anterior (Fig. S5b), and for an increase in the expression of atrogin-1 (Fig. 5g) and MuRF-1 (Fig. 5h). Together, our results suggest that Mas expression is increased under conditions of skeletal muscle atrophy by immobilization concomitant with typical parameters of skeletal muscle atrophy.
Mas receptor is upregulated in disuse-induced skeletal muscle atrophy. Skeletal muscle atrophy was induced in C57BL/10J mice by unilateral hindlimb immobilization by casting. a Mas receptor expression from gastrocnemius non-immobilizated (NI) and immobilizated (I) hindlimb for 24 h was determined by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control muscles. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the control muscles). b Mas receptor protein levels were evaluated by Western blot in gastrocnemius of non-immobilizated and immobilizated hindlimb for 14 days. Tubulin is shown as the loading control. Molecular weights are indicated in kilodaltons (kDa). c Quantification of B. Values correspond to the mean ± SD of three independent experiments and are expressed as fold of induction relative to the expression in non-immobilizated gastrocnemius (NI) (*P < 0.05). d Mas receptor was detected by indirect immunohistochemical analysis in cryosections of gastrocnemius muscles from non-immobilizated and immobilizated hindlimb for 14 days. Nuclei were labelled with haematoxylin. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. Upper ×10 magnification; lower ×40 magnification. e Histological analysis of non-immobilizated and immobilizated hindlimb for 14 days. Muscle cross sections were stained with haematoxylin and eosin to visualize muscle architecture. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. Upper ×10 magnification; lower ×40 magnification. f Quantitative analysis of the fibre diameter from three independent experiments using three mice for each experimental condition. The values are expressed as the percentage of the total fibres quantified (*P < 0.05 relative to non-immobilizated muscles). g Atrogin-1 and h MuRF-1 ubiquitin ligase mRNA levels from gastrocnemius of non-immobilizated and immobilizated hindlimb for 18 h were determined by RT-qPCR using β-actin as the reference gene. The levels were expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*P < 0.05 relative to the non-immobilizated muscles)
Discussion
In this study, we show for the first time that the levels of Mas receptor for Ang (1–7) are regulated in skeletal muscle under atrophic conditions. We found that Mas expression in mRNA and protein levels increases in response to AngII, LPS, and immobilization together with the upregulation of typical parameters of skeletal muscle atrophy, such as the muscle-specific E3 ubiquitin ligases atrogin-1 and MuRF-1, or the decrease in fibre diameter.
The three different models of skeletal muscle atrophy used for us developed typical features of muscle wasting such as increase of UPS activity (specifically the increase in the expression of atrogin-1 and MuRF-1) and decrease of fibre size. Several signalling pathways dependent on AKT, reactive oxygen species (ROS), and p38 MAPK are involved in the regulation of the atrogin-1 and MuRF-1 expression (Foletta et al. 2011). Thus, it has been reported that AKT phosphorylation is key to prevent the induction of atrogin-1 gene expression by regulating FOXO activity (Foletta et al. 2011; Stitt et al. 2004). ROS is directly involved in the muscle proteolysis and in the increase of UPS in muscle wasting induced by AngII, immobilization, and LPS (Kondo et al. 1993, 1994; Powers et al. 2007, 2012; Sukhanov et al. 2011; Tisdale 2005; Yu et al. 2008). We have previously reported that AngII induces ROS in skeletal muscle cells through a mechanism dependent on NADPH oxidase which is decreased by Ang (1–7) (Morales et al. 2014). Furthermore, p38 MAPK has been demonstrated to be a key signalling in the induction of skeletal muscle atrophy, also in the increase of atrogin-1 and MuRF-1 by oxidative stress (Doyle et al. 2011; Glass 2005; McClung et al. 2010).
Several studies have shown the importance of the balance between the classical and non-classical RAS axes. In this context, our results showed that Mas, the main receptor of the non-classical RAS axis associated with beneficial effects in many tissues including skeletal muscle, is increased in skeletal muscle wasting. It has been reported that LPS induces the Mas upregulation in macrophages (Souza and Costa-Neto 2012) which is in agreement with our findings in skeletal muscle. Regarding to immobilization and AngII treatment, there are not reports showing increase of the Mas receptor levels. In general, the expression of Mas has been reported to be downregulated under some pathological conditions: in the dorsal medulla by exposure to glucocorticoid (Marshall et al. 2013), in cardiomyocytes by endothelin-1 (Chen et al. 2013), in myocardial infarction, cardiac hypertrophy, and damage elicited by isoproterenol treatment (Dias-Peixoto et al. 2012). Despite the most of the evidence suggests a decrease of Ang (1–7)/Mas axis in pathological status in which RAS is involved, there are some exceptions. Mas expression increases in ovary under treatment with chorionic gonadotropin (Pereira et al. 2009), in brain under acute ischaemic stroke (Lu et al. 2013), in heart of DOCA-salt rats (Dias-Peixoto et al. 2012), in hypertensive rats subjected to swimming training (Filho et al. 2008), in gastrointestinal smooth muscles of patients with achalasia (Casselbrant et al. 2014), in jejunal enterocytes from diabetic mellitus type 1 (Wong et al. 2012), and in severe acute pancreatitis (Wang et al. 2012). Thus, our study is not the only evidence of the Mas upregulation under pathological conditions.
There are few evidences about molecular mechanisms related to the increase of Mas gene expression. A recent study shows a mechanism involved in the epigenetic regulation of the Mas gene through nitration of ZNF274/KAP-1 (KRAB-associated protein-1) protein complex (Prokop et al. 2014). Further experiments could be performed to evaluate this regulatory mechanism of Mas receptor gene expression in skeletal muscle atrophy. In our study, we not have elucidated the signalling pathways involved in the increase of Mas reported in this work under different atrophic stimuli. One possibility is that the increase of Mas levels in vivo with could be explained as an indirect effect dependent on IL-1β production, which is increased by AngII, LPS and immobilization (Borge et al. 2009; Bruells et al. 2013; Cabello-Verrugio et al. 2012a; Wang et al. 2014) and upregulates Mas mRNA and protein expression (Ender et al. 2014). Another possibility is the participation of p38MAPK that is has been recently described to be involved in the transcriptional upregulation of Mas in dorsal root ganglia neurons (Cao et al. 2013). Interestingly, p38 MAPK has been found to be a key player in skeletal muscle atrophy induced by immobilization, AngII treatment and LPS (Doyle et al. 2011; Eley et al. 2008; Kim et al. 2009). Thus, we can speculate that p38MAPK could participate of the Mas upregulation in the atrophic models used for us. Thus, further studies must be performed to evaluate the role of p38 MAPK in this process.
Our results show that Mas is located at least, in the plasma membrane of muscle fibres of the gastrocnemius and tibialis anterior, which indicate that muscle fibres have the ability to respond to Ang (1–7). This fact can have high relevance to evaluate the effect of Ang (1–7) administration on the weakness associated with skeletal muscle atrophy. A pathological condition in which skeletal muscle atrophy is observed is the dystrophic muscles of aged mdx mice (Mouisel et al. 2010). Recent reports have shown a decrease in fibrosis and damage of the skeletal muscles of dystrophic mice treated with Ang (1–7) which are mediated through the Mas receptor (Acuna et al. 2014; Sabharwal et al. 2014). In this context, it is important to determine the signalling pathway activated by Ang (1–7) through Mas that could be involved in a possible effect of Ang (1–7) on skeletal muscle atrophy. Ang (1–7) binds to the Mas receptor which activates intracellular signalling pathways through nitric oxide synthase and AKT (Sampaio et al. 2007), GSK3β (Gomes et al. 2010), SHP-1 (Gava et al. 2009), or by inhibition of NF-κBeta pathway (El-Hashim et al. 2012; Jiang et al. 2012; Santos et al. 2013). In regard to skeletal muscle atrophy, AKT signalling is decreased which activates FOXO proteins and produces the increment of the atrogin-1 and MuRF-1 gene expression (Sandri et al. 2004). In this context, we have data that demonstrate Ang (1–7) has an anti-atrophic effect on AngII-induced muscle wasting through the AKT phosphorylation (data not shown). It has been reported that Ang (1–7) can modulate the NF-κBeta signalling in liver, brain, and lung (El-Hashim et al. 2012; Jiang et al. 2012; Santos et al. 2013). Thus, it would be important to evaluate the effect of Ang (1–7) on the NF-κBeta signalling pathway because it, among others, is directly involved in the modulation of MuRF-1 gene expression.
Our study demonstrates for the first time that the expression of the Mas receptor is modulated under several conditions of muscle wasting and increases the knowledge describing a differential expression of a Mas receptor that might be involved in the pathophysiology of skeletal muscle atrophy.
References
Acuna MJ et al (2014) Restoration of muscle strength in dystrophic muscle by angiotensin-1–7 through inhibition of TGF-beta signalling. Hum Mol Genet 23:1237–1249
Agarwal R (2003) Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am J Physiol Renal Physiol 284:F863–F869
Agarwal R, Campbell RC, Warnock DG (2004) Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol 24:101–114
Antoniu SA (2008) Targeting the angiotensin pathway in idiopathic pulmonary fibrosis. Expert Opin Ther Targets 12:1587–1590
Benter IF, Ferrario CM, Morris M, Diz DI (1995) Antihypertensive actions of angiotensin-(1–7) in spontaneously hypertensive rats. Am J Physiol 269:H313–H319
Bodine SC et al (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Borge BA, Kalland KH, Olsen S, Bletsa A, Berggreen E, Wiig H (2009) Cytokines are produced locally by myocytes in rat skeletal muscle during endotoxemia. Am J Physiol Heart Circ Physiol 296:H735–H744
Brecher P (1996) Angiotensin II and cardiac fibrosis. Trends Cardiovasc Med 6:193–198
Bruells CS et al (2013) Prolonged mechanical ventilation alters the expression pattern of angio-neogenetic factors in a pre-clinical rat model. PLoS ONE 8:e70524
Cabello-Verrugio C, Acuna MJ, Morales MG, Becerra A, Simon F, Brandan E (2011) Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem Biophys Res Commun 410:665–670
Cabello-Verrugio C, Cordova G, Salas JD (2012a) Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci 13:560–569
Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E (2012b) Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med 16:752–764
Cao L, Xun J, Jiang X, Tan R (2013) Propofol up-regulates Mas receptor expression in dorsal root ganglion neurons. Pharmazie 68:677–680
Casselbrant A, Kostic S, Lonroth H (2014) The muscular expression of RAS in patients with achalasia. J Renin Angiotensin Aldosterone Syst. doi:10.1177/1470320313498294
Chappell MC (2007) Emerging evidence for a functional angiotensin-converting enzyme 2–angiotensin-(1–7)–MAS receptor axis: more than regulation of blood pressure? Hypertension 50:596–599
Chen Z, Tang Y, Yang Z, Liu S, Liu Y, Li Y, He W (2013) Endothelin-1 downregulates Mas receptor expression in human cardiomyocytes. Mol Med Rep 8:871–876
Dias-Peixoto MF et al (2012) The cardiac expression of Mas receptor is responsive to different physiological and pathological stimuli. Peptides 35:196–201
Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP (2011) Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin–proteasome and autophagy–lysosome pathways. FASEB J 25:99–110
Echeverria-Rodriguez O, Del Valle-Mondragon L, Hong E (2013) Angiotensin 1–7 improves insulin sensitivity by increasing skeletal muscle glucose uptake in vivo. Peptides 51:26–30
Eley HL, Russell ST, Tisdale MJ (2008) Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab 295:E1409–E1416
El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF (2012) Angiotensin-(1–7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kappaB-dependent pathways. Br J Pharmacol 166:1964–1976
Ender SA, Dallmer A, Lassig F, Lendeckel U, Wolke C (2014) Expression and function of the ACE2/angiotensin(1–7)/Mas axis in osteosarcoma cell lines U-2 OS and MNNG-HOS. Mol Med Rep 10:804–810. doi:10.3892/mmr.2014.2266
Ferrario CM, Trask AJ, Jessup JA (2005) Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289:H2281–H2290
Ferreira AJ, Bader M, Santos RA (2012a) Therapeutic targeting of the angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas cascade in the renin–angiotensin system: a patent review. Expert Opin Ther Pat 22:567–574
Ferreira AJ, Murca TM, Fraga-Silva RA, Castro CH, Raizada MK, Santos RA (2012b) New cardiovascular and pulmonary therapeutic strategies based on the angiotensin-converting enzyme 2/angiotensin-(1–7)/mas receptor axis Int. J Hypertens 2012:147825
Filho AG et al (2008) Selective increase of angiotensin(1–7) and its receptor in hearts of spontaneously hypertensive rats subjected to physical training. Exp Physiol 93:589–598
Foletta VC, White LJ, Larsen AE, Leger B, Russell AP (2011) The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch 461:325–335
Fyhrquist F, Saijonmaa O (2008) Renin–angiotensin system revisited. J Intern Med 264:224–236
Gava E et al (2009) Angiotensin-(1–7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrol Dial Transplant 24:1766–1773
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Trends Mol Med 9:344–350
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98:14440–14445
Gomes ER et al (2010) Angiotensin-(1–7) prevents cardiomyocyte pathological remodeling through a nitric oxide/guanosine 3′,5′-cyclic monophosphate-dependent pathway. Hypertension 55:153–160
Grobe JL, Mecca AP, Mao H, Katovich MJ (2006) Chronic angiotensin-(1–7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol 290:H2417–H2423
Gumucio JP, Mendias CL (2013) Atrogin-1, MuRF-1, and sarcopenia. Endocrine 43:12–21
Iwai M, Horiuchi M (2009) Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1–7)–Mas receptor axis. Hypertens Res 32:533–536
Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH (2005) Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 289:H2356–H2363
Jiang T, Gao L, Guo J, Lu J, Wang Y, Zhang Y (2012) Suppressing inflammation by inhibiting the NF-kappaB pathway contributes to the neuroprotective effect of angiotensin-(1–7) in rats with permanent cerebral ischaemia. Br J Pharmacol 167:1520–1532
Kim J et al (2009) p38 MAPK participates in muscle-specific RING finger 1-mediated atrophy in cast-immobilized rat gastrocnemius muscle. Korean J Physiol Pharmacol 13:491–496
Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y (1993) Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol 265:E839–E844
Kondo H, Nishino K, Itokawa Y (1994) Hydroxyl radical generation in skeletal muscle atrophied by immobilization. FEBS Lett 349:169–172
Lu J et al (2013) The expression of angiotensin-converting enzyme 2–angiotensin-(1–7)–Mas receptor axis are upregulated after acute cerebral ischemic stroke in rats. Neuropeptides 47:289–295
Lubel JS, Herath CB, Burrell LM, Angus PW (2008) Liver disease and the renin–angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol 23:1327–1338
Madaro L, Smeriglio P, Molinaro M, Bouché M (2008) Unilateral immobilization: a simple model of limb atrophy in mice. Basic Appl Myol 18:149–153
Mancini GB, Khalil N (2005) Angiotensin II type 1 receptor blocker inhibits pulmonary injury. Clin Invest Med 28:118–126
Marangoni RA, Carmona AK, Passaglia RC, Nigro D, Fortes ZB, de Carvalho MH (2006) Role of the kallikrein–kinin system in Ang-(1–7)-induced vasodilation in mesenteric arterioles of Wistar rats studied in vivo-in situ. Peptides 27:1770–1775
Marshall AC, Shaltout HA, Nautiyal M, Rose JC, Chappell MC, Diz DI (2013) Fetal betamethasone exposure attenuates angiotensin-(1–7)–Mas receptor expression in the dorsal medulla of adult sheep. Peptides 44:25–31
McClung JM, Judge AR, Powers SK, Yan Z (2010) p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298:C542–C549
Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D (1995) Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett 357:27–32
Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E (2011) CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol 225:490–501
Morales MG et al (2012) Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol 44:1993–2002
Morales MG, Cabrera D, Cespedes C, Vio CP, Vazquez Y, Brandan E, Cabello-Verrugio C (2013a) Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2). Cell Tissue Res 353:173–187
Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E (2013b) Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet 22:4938–4951
Morales MG et al (2014) The Ang-(1–7)/Mas-1 axis attenuates the expression and signalling of TGF-beta1 induced by AngII in mouse skeletal muscle. Clin Sci (Lond) 127:251–264
Mouisel E, Vignaud A, Hourde C, Butler-Browne G, Ferry A (2010) Muscle weakness and atrophy are associated with decreased regenerative capacity and changes in mTOR signaling in skeletal muscles of venerable (18–24-month-old) dystrophic mdx mice. Muscle Nerve 41:809–818
Munoz MC, Giani JF, Dominici FP (2010) Angiotensin-(1–7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept 161:1–7
Munoz MC, Giani JF, Burghi V, Mayer MA, Carranza A, Taira CA, Dominici FP (2012) The Mas receptor mediates modulation of insulin signaling by angiotensin-(1–7). Regul Pept 177:1–11
Painemal P, Acuna MJ, Riquelme C, Brandan E, Cabello-Verrugio C (2013) Transforming growth factor type beta 1 increases the expression of angiotensin II receptor type 2 by a SMAD- and p38 MAPK-dependent mechanism in skeletal muscle. BioFactors 39:467–475
Pereira VM, Reis FM, Santos RA, Cassali GD, Santos SH, Honorato-Sampaio K, dos Reis AM (2009) Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod Sci 16:1165–1174
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102:2389–2397
Powers SK, Smuder AJ, Judge AR (2012) Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care 15:240–245
Prasannarong M, Santos FR, Henriksen EJ (2012) ANG-(1–7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem Biophys Res Commun 426:369–373
Prokop JW et al (2014) MAS promoter regulation: a role for Sry and tyrosine nitration of the KRAB domain of ZNF274 as a feedback mechanism. Clin Sci (Lond) 126:727–738
Rezk BM, Yoshida T, Semprun-Prieto L, Higashi Y, Sukhanov S, Delafontaine P (2012) Angiotensin II infusion induces marked diaphragmatic skeletal muscle atrophy. PLoS ONE 7:e30276
Sabharwal R, Cicha MZ, Sinisterra RD, De Sousa FB, Santos RA, Chapleau MW (2014) Chronic oral administration of Ang-(1–7) improves skeletal muscle, autonomic and locomotor phenotypes in muscular dystrophy. Clin Sci (Lond) 127:101–109
Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM (2007) Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49:185–192
Sanders PM, Russell ST, Tisdale MJ (2005) Angiotensin II directly induces muscle protein catabolism through the ubiquitin–proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 93:425–434
Sandri M et al (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Santos RA et al (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100:8258–8263
Santos SH et al (2013) Oral Angiotensin-(1–7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides 46:47–52
Semprun-Prieto LC et al (2011) Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409:217–221
Souza LL, Costa-Neto CM (2012) Angiotensin-(1–7) decreases LPS-induced inflammatory response in macrophages. J Cell Physiol 227:2117–2122
Stitt TN et al (2004) The IGF-1/PI3 K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P (2011) Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 342:143–147
Tallant EA, Ferrario CM, Gallagher PE (2005) Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289:H1560–H1566
Tisdale MJ (2005) The ubiquitin–proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol 3:209–217
Villar AJ, Pedersen RA (1994) Parental imprinting of the Mas protooncogene in mouse. Nat Genet 8:373–379
Wang Y, Wang J, Liu R, Qi H, Wen Y, Sun F, Yin C (2012) Severe acute pancreatitis is associated with upregulation of the ACE2–angiotensin-(1–7)–Mas axis and promotes increased circulating angiotensin-(1–7). Pancreatology 12:451–457
Wang Y et al (2014) TNF-alpha and IL-1beta neutralization ameliorates angiotensin II-induced cardiac damage in male mice. Endocrinology 155:2677–2687. doi:10.1210/en.2013-2065
Wong TP, Ho KY, Ng EK, Debnam ES, Leung PS (2012) Upregulation of ACE2–ANG-(1–7)–Mas axis in jejunal enterocytes of type 1 diabetic rats: implications for glucose transport. Am J Physiol Endocrinol Metab 303:E669–E681
Yu Z, Li P, Zhang M, Hannink M, Stamler JS, Yan Z (2008) Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS ONE 3:e2086
Acknowledgments
This study was supported by research grants from Association-Francaise Contre Les Myopathies AFM 16670 (CCV); FONDECYT 1120380 (CCV), 3130593 (MGM), 1121078 (FS); and the Millennium Institute on Immunology and Immunotherapy, P09-016-F (FS); UNAB-DI-281-13/R (CCV).
Author information
Authors and Affiliations
Corresponding author
Additional information
María Gabriela Morales and Johanna Abrigo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2014_1275_MOESM1_ESM.tif
Fig. S1: Upregulation of the Mas receptor induced by AngII in C 2 C 12 myotubes is dependent on the AT-1 receptor. C2C12 myotubes from day 5 were pre-incubated with losartan (10 μM) or PD123319 (10 μM) for 1 h prior to the incubation with AngII (500 nM) for 3 h. At the end of this treatment, the mRNA levels of Mas were determined by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*, P < 0.05 relative to the control cells, #, P < 0.05 relative to AngII-vehicle) (TIFF 219 kb)
418_2014_1275_MOESM2_ESM.tif
Fig. S2: The Mas receptor is increased in the tibialis anterior of AngII-induced skeletal muscle atrophy. Skeletal muscle atrophy was induced in C57BL/10J mice by osmotic infusion of AngII (1 μg/kg/min). (a) Mas receptor was detected by indirect immunohistochemical analysis in cryosections of tibialis anterior muscles from control mice and mice treated with AngII for 14 days. Nuclei were labelled with haematoxylin. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. (b) Histological analysis of the tibialis anterior of control mice and mice treated with AngII for 14 days. Muscle cross sections were stained with haematoxylin and eosin to visualize muscle architecture. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. (c) Quantitative analysis of the fibre diameter from three independent experiments using three mice for each experimental condition. The values are expressed as the percentage of the total fibres quantified (*, P < 0.05 relative to the control) (TIFF 8578 kb)
418_2014_1275_MOESM3_ESM.tif
Fig. S3: Upregulation of Mas receptor induced by LPS in C 2 C 12 myotubes is dependent on the TLR-4 receptor. C2C12 myotubes from day 5 were pre-incubated with CLI-095 (5 μM) for 1 h prior to the incubation with LPS (500 ng/ml) for 3 h. At the end of this treatment, mRNA levels of Mas were determined by RT-qPCR using β-actin as the reference gene. The expression was expressed as the fold of induction, normalized to the levels in the control cells. Values correspond to the mean ± SD of three independent experiments (*, P < 0.05 relative to the control cells, #, P < 0.05 relative to LPS-vehicle) (TIFF 208 kb)
418_2014_1275_MOESM4_ESM.tif
Fig. S4: The Mas receptor is upregulated in the tibialis anterior of LPS-induced skeletal muscle atrophy. Skeletal muscle atrophy was induced in C57BL/10J mice by intraperitoneal injection of LPS (1 mg/kg). (a) The Mas receptor was detected by indirect immunohistochemical analysis in cryosections of the tibialis anterior of muscles from control and LPS-treated mice for 14 days. Nuclei were labelled with haematoxylin. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. (b) Histological analysis of the tibialis anterior of control mice and mice treated with LPS for 14 days. Muscle cross sections were stained with haematoxylin and eosin to visualize muscle architecture. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. (c) Quantitative analysis of the fibre diameter from three independent experiments using three mice for each experimental condition. The values are expressed as the percentage of the total fibres quantified (*, P < 0.05 relative to the control) (TIFF 8007 kb)
418_2014_1275_MOESM5_ESM.tif
Fig. S5: The Mas receptor is augmented in the tibialis anterior of disuse-induced skeletal muscle atrophy. Skeletal muscle atrophy was induced in C57BL/10J mice by unilateral immobilizated hindlimb by casting. (a) The Mas receptor was detected by indirect immunohistochemical analysis in cryosections of tibialis anterior muscles from non-immobilized hindlimbs and hindlimbs immobilized for 14 days. Nuclei were labelled with haematoxylin. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. (b) Histological analysis of tibialis anterior non-immobilized hindlimbs and hindlimbs immobilized for 14 days. Muscle cross sections were stained with haematoxylin and eosin to visualize muscle architecture. The bar corresponds to 50 μm. The images are representative of three independent experiments, using three mice for each experimental condition. (c) Quantitative analysis of the fibre diameter from three independent experiments using three mice for each experimental condition. The values are expressed as the percentage of the total fibres quantified (*, P < 0.05 relative to the non-immobilizated muscles) (TIFF 8431 kb)
Rights and permissions
About this article
Cite this article
Morales, M.G., Abrigo, J., Meneses, C. et al. Expression of the Mas receptor is upregulated in skeletal muscle wasting. Histochem Cell Biol 143, 131–141 (2015). https://doi.org/10.1007/s00418-014-1275-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1275-1