Abstract
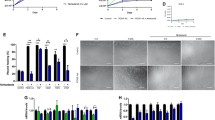
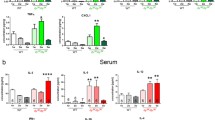
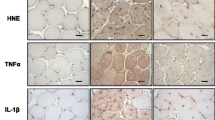
The renin-angiotensin system (RAS), through angiotensin II and the angiotensin-converting enzyme (ACE), is involved in the genesis and progression of fibrotic diseases characterized by the replacement of normal tissue by an accumulation of an extracellular matrix (ECM). Duchenne muscular dystrophy (DMD) presents fibrosis and a decrease in muscle strength produced by chronic damage. The mdx mouse is a murine model of DMD and develops the same characteristics as dystrophic patients when subjected to chronic exercise. The connective tissue growth factor (CTGF/CCN2) and transforming growth factor type beta (TGF-β), which are overexpressed in muscular dystrophies, play a major role in many progressive scarring conditions. We have tested the hypothesis that ACE inhibition decreases fibrosis in dystrophic skeletal muscle by treatment of mdx mice with the ACE inhibitor enalapril. Both sedentary and exercised mdx mice treated with enalapril showed improvement in gastrocnemius muscle strength explained by a reduction in both muscle damage and ECM accumulation. ACE inhibition decreased CTGF expression in sedentary or exercised mdx mice and diminished CTGF-induced pro-fibrotic activity in a model of CTGF overexpression by adenoviral infection. Enalapril did not have an effect on TGF-β1 expression or its signaling activity in sedentary or exercised dystrophic mice. Thus, ACE inhibition might improve muscle strength and decrease fibrosis by diminishing specifically CTGF expression and activity without affecting TGF-β1 signaling. Our data provide insights into the pathogenic events in dystrophic muscle. We propose ACE as a target for developing therapies for DMD and related diseases.









Similar content being viewed by others
Abbreviations
- Ang-II:
-
Angiotensin II
- ACE:
-
Angiotensin-converting enzyme
- ARB:
-
Angiotensin II receptor type 1 blocker
- AT-1 receptor:
-
Angiotensin II receptor type I
- CTGF:
-
Connective tissue growth factor
- DMD:
-
Duchenne muscular dystrophy
- ECM:
-
Extracellular matrix
- mdx :
-
DMD mouse model
- RAS:
-
Renin angiotensin system
- TGF-β:
-
Transforming growth factor beta
References
Abraham D (2008) Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford) 47 (Suppl 5):v8–v9
Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P (2006) Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J Neuroimmunol 175:77–86
Arthur PG, Grounds MD, Shavlakadze T (2008) Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care 11:408–416
Bauer R, Straub V, Blain A, Bushby K, MacGowan GA (2009) Contrasting effects of steroids and angiotensin-converting-enzyme inhibitors in a mouse model of dystrophin-deficient cardiomyopathy. Eur J Heart Fail 11:463–471
Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R (1999) Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord 9:28–33
Bish LT, Yarchoan M, Sleeper MM, Gazzara JA, Morine KJ, Acosta P, Barton ER, Sweeney HL (2011) Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One 6:e20856
Bizario JC, Cerri DG, Rodrigues LC, Oliveira GL, Nomizo A, de Araujo DD, Fukuhara PS, Ribeiro JC, de Castro FA, Costa MC (2009) Imatinib mesylate ameliorates the dystrophic phenotype in exercised mdx mice. J Neuroimmunol 212:93–101
Brussee V, Tardif F, Tremblay JP (1997) Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord 7:487–492
Burdi R, Rolland JF, Fraysse B, Litvinova K, Cozzoli A, Giannuzzi V, Liantonio A, Camerino GM, Sblendorio V, Capogrosso RF, Palmieri B, Andreetta F, Confalonieri P, De Benedictis L, Montagnani M, De Luca A (2009) Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: outcome of a large array of in vivo and ex vivo tests. J Appl Physiol 106:1311–1324
Cabello-Verrugio C, Acuna MJ, Morales MG, Becerra A, Simon F, Brandan E (2011) Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem Biophys Res Commun 410:665–670
Cabello-Verrugio C, Cordova G, Salas JD (2012a) Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci 13:560–569
Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E (2012b) Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med 16:752–764
Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC (2007) Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13:204–210
Cozzoli A, Nico B, Sblendorio VT, Capogrosso RF, Dinardo MM, Longo V, Gagliardi S, Montagnani M, De Luca A (2011) Enalapril treatment discloses an early role of angiotensin II in inflammation- and oxidative stress-related muscle damage in dystrophic mdx mice. Pharmacol Res 64:482–492
De Luca A, Pierno S, Liantonio A, Cetrone M, Camerino C, Fraysse B, Mirabella M, Servidei S, Ruegg UT, Conte Camerino D (2003) Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J Pharmacol Exp Ther 304:453–463
De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, Burdi R, Mangieri D, Rolland JF, Camerino C, Zallone A, Confalonieri P, Andreetta F, Arnoldi E, Courdier-Fruh I, Magyar JP, Frigeri A, Pisoni M, Svelto M, Conte Camerino D (2005) A multidisciplinary evaluation of the effectiveness of cyclosporine a in dystrophic mdx mice. Am J Pathol 166:477–489
De Luca A, Nico B, Rolland JF, Cozzoli A, Burdi R, Mangieri D, Giannuzzi V, Liantonio A, Cippone V, De Bellis M, Nicchia GP, Camerino GM, Frigeri A, Svelto M, Camerino DC (2008) Gentamicin treatment in exercised mdx mice: identification of dystrophin-sensitive pathways and evaluation of efficacy in work-loaded dystrophic muscle. Neurobiol Dis 32:243–253
Goldstein JA, McNally EM (2010) Mechanisms of muscle weakness in muscular dystrophy. J Gen Physiol 136:29–34
Graham KM, Singh R, Millman G, Malnassy G, Gatti F, Bruemmer K, Stefanski C, Curtis H, Sesti J, Carlson CG (2010) Excessive collagen accumulation in dystrophic (mdx) respiratory musculature is independent of enhanced activation of the NF-kappaB pathway. J Neurol Sci 294:43–50
Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS (2002) Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol 161:2263–2272
Iwai M, Horiuchi M (2009) Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens Res 32:533–536
Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA (2008) Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve 38:1290–1303
Leask A (2007) TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 74:207–212
Marshall PA, Williams PE, Goldspink G (1989) Accumulation of collagen and altered fiber-type ratios as indicators of abnormal muscle gene expression in the mdx dystrophic mouse. Muscle Nerve 12:528–537
Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E (2011) CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol 225:490–501
Morales M, Vazquez Y, Acuña M, Rivera J, Simon F, Salas J, Álvarez-Ruf J, Brandan E, Cabello-Verrugio C (2012) Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol 44:1993–2002
Moyer AL, Wagner KR (2010) Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol 23:568–573
Okano T, Yoshida K, Nakamura A, Sasazawa F, Oide T, Takeda S, Ikeda S (2005) Chronic exercise accelerates the degeneration-regeneration cycle and downregulates insulin-like growth factor-1 in muscle of mdx mice. Muscle Nerve 32:191–199
Pereira RM, dos Santos RA, da Costa Dias FL, Teixeira MM, Simoes e Silva AC (2009) Renin-angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol 15:2579–2586
Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G (2009) TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta 1792:746–756
Salas SP, Giacaman A, Vio CP (2004) Renal and hormonal effects of water deprivation in late-term pregnant rats. Hypertension 44:334–339
Sandri M, Podhorska-Okolow M, Geromel V, Rizzi C, Arslan P, Franceschi C, Carraro U (1997) Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol 56:45–57
Sun G, Haginoya K, Dai H, Chiba Y, Uematsu M, Hino-Fukuyo N, Onuma A, Iinuma K, Tsuchiya S (2009) Intramuscular renin-angiotensin system is activated in human muscular dystrophy. J Neurol Sci 280:40–48
Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, Sato Y, Takigawa M, Iinuma K, Tsuchiya S (2008) Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci 267:48–56
Vial C, Zuniga LM, Cabello-Verrugio C, Canon P, Fadic R, Brandan E (2008) Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol 215:410–421
Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP (1998) Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve 21:567–576
Wang P, Fedoruk MN, Rupert JL (2008) Keeping pace with ACE: are ACE inhibitors and angiotensin II type 1 receptor antagonists potential doping agents? Sports Med 38:1065–1079
Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS (2006) Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281:35137–35146
Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS (2008) Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab 294:E345–E351
Weller B, Karpati G, Carpenter S (1990) Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci 100:9–13
Whitehead NP, Yeung EW, Froehner SC, Allen DG (2010) Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One 5:e15354
Zeman RJ, Peng H, Danon MJ, Etlinger JD (2000) Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve 23:521–528
Zhou L, Lu H (2010) Targeting fibrosis in Duchenne muscular dystrophy. J Neuropathol Exp Neurol 69:771–776
Zhou L, Porter JD, Cheng G, Gong B, Hatala DA, Merriam AP, Zhou X, Rafael JA, Kaminski HJ (2006) Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul Disord 16:32–38
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by research grants from Association-Francaise Contre Les Myopathies AFM 16670 (C.C.-V.); FONDECYT 1120380 (C.C.-V.), 11080212 (C.C.-V.), 1110426 (E.B.), 3130593 (M.G.M.); UNAB-DI-281-13/R (C.C.-V.); CARE PFB12/2007 (E.B., C.P.V.); CONICYT AT-24100047 (M.G.M.), AT-24090192 (D.C.) and Fundación Chilena para Biología Celular Proyecto MF-100 (E.B.).
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Improvement of skeletal muscle strength by enalapril in sedentary and exercised mdx mice. Tetanic specific force of normal, sedentary (a), or exercised (b) mdx mice treated with a vehicle (Vehicle), enalapril (Enal), or losartan (Los). Values are represented as percentages of specific isometric force (mN/mm2) shown by normal muscles (*,# P<0.05). (DOC 404 kb)
Fig. S2
Enalapril treatment does not change the number of central nuclei but decreases fibrosis in dystrophic skeletal muscle (Enal enalapril, Los losartan). a Central nuclei of the fibers were measured as indicated in Material and Methods. Values correspond to the means ± SD of three independent experiments with four mice for each experimental condition. b Fibrotic area (% of total) measured by using immunofluorescence for fibronectin (shown in Figure 4) as described in Materials and Methods. Values correspond to the means ± SD of three independent experiments with four mice for each experimental condition (*,**,#,## P<0.05). (DOC 468 kb)
Fig. S3
ACE inhibition decreases the angiotensin II signaling pathway in skeletal muscle that overexpress CTGF. Tibialis anterior (TA) of normal mice systemically treated with a vehicle (Veh), enalapril (Enal), or losartan (Los) were infected with a control adenovirus (Adv-Ctrl) or with an adenovirus to overexpress CTGF (Adv-CTGF). Phospho-p38 and total p38 protein levels were detected by Western blot analysis in extracts obtained from TA muscles. Molecular weights are shown in kDa. (DOC 389 kb)
Fig. S4
Extracellular signal-related kinase (ERK) phosphorylation is decreased by enalapril in sedentary and exercised mdx mice. a Protein levels of phospho-ERK and total ERK-1/2 were detected by Western blot analysis in extracts obtained from the gastrocnemius muscles of normal and of sedentary and exercised mdx mice treated with a vehicle or enalapril for 6 months. Molecular weight markers are shown in kDa. b Densitometric analysis of Western blots shown in a. Values correspond to means ± SD of three independent experiments with five mice for each experimental condition (*P<0.05). (DOC 552 kb)
Rights and permissions
About this article
Cite this article
Morales, M.G., Cabrera, D., Céspedes, C. et al. Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2). Cell Tissue Res 353, 173–187 (2013). https://doi.org/10.1007/s00441-013-1642-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1642-6