Abstract
Purpose
Bilateral pediatric cataract (BPC) is one of the most common causes of childhood visual impairment and blindness worldwide. A significant percentage of pediatric cataracts are caused by genetic alterations. We aim to characterize the set of genes and variants that cause BPC in the Israeli and Palestinian populations and to assess genotype-phenotype correlation.
Methods
Retrospective study in a multidisciplinary center for visual impairment, located in a tertiary medical center. Medical charts of families who underwent genetic counseling because of BPC in a family member were reviewed. Clinical characteristics and genetic tests results were obtained from medical records of affected subjects.
Results
Twenty-two families (47 patients) underwent genetic counseling and completed genetic testing. Causative variants were identified in 18/22 (81.8%) of the families, including 3 novel variants. Genetic testing used included mainly panel for congenital cataracts and whole exome sequencing. Eleven families performed genetic testing with the intention of future pregnancy planning. Main causative genes identified were crystalline genes followed by transcription factor genes. BCOR gene variants were associated with persistent fetal vasculature (PFV) cataract in two of three families.
Conclusions
Combined molecular techniques are useful in identifying variants causing pediatric cataracts and showed a high detection rate in our population. BCOR gene variants might be associated with PFV type of cataracts. The study of pathogenic variants may aid in family planning and prevention of pediatric cataracts in future pregnancies. Additionally, in certain cases, it assists in diagnosing non-suspected syndromic types of pediatric cataracts.
Key message
What is known
• Bilateral pediatric cataracts are often inherited.
• Detection rate of causative variants is high.
• Correlation between genes and phenotype is still scarce.
What is new
• First evidence of possible correlation between BCOR gene and persistent fetal vasculature cataract.
• Additional data supporting recently published relationship between GJA3 gene variant (c.199G > T) and glaucoma.
• Importance of genetic testing in diagnosing “non-suspected syndromic types of pediatric cataracts”.
• Three novel causative variants identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bilateral pediatric cataract (BPC) is a common cause for childhood blindness accounting for 10-to-20% of reported cases worldwide [1]. Although BPC is a relatively rare condition, it can cause severe visual impairment in around 40% of cases even when treated timely with modern surgical techniques [2].
Based on a systematic review the prevalence of cataracts in childhood was estimated to range from 1.91 to 4.24 per 10,000, with the highest numbers in Asia reaching 7.43 per 10,000 [3]. In the United kingdom prevalence was reported as 3.18 per 10,000 by 5 years of age [4].
BPC may occur as an isolated pathology, may be associated with other ocular developmental abnormalities, or as part of a systemic condition [3, 5, 6]. Approximately 25-50% of the pediatric cataracts are inherited [3, 7]. The majority of the inherited cataracts are autosomal dominant while autosomal recessive and X-linked inheritance patterns have also been reported [8, 9]. Up-to date approximately 100 genes and around 200 loci have been identified as causatives of BPC (“Cat-Map” website (http://cat-map.wustl.edu/) [5, 9], suggesting the lack of a main gene accounting for a high percentage of cases [8].
Attempts have been made to find a phenotype that points to a specific affected gene, but similar cataract morphologies can be associated with different gene variants and different cataract types can be identified in a given gene variant, thus limiting efforts to make informative genotype-phenotype correlations [5].
The detection rate for genetic variants has been reported to be around 75%, based on gene panels using next generation sequencing (NGS) [10].
The aim of our study was to report the variety of gene variants (novel and recurrent) causing BPC and the detection rate for these variants in our population. We also aimed to look for any correlations between cataract characteristics and the causing gene variants.
Methods
Data were collected from medical charts of patients seen in a low vision clinic located at a tertiary University Hospital. This multidisciplinary clinic includes pediatric ophthalmologists, low vision optometrists, a social worker and genetic counselors specialized in inherited eye diseases (IED). Patients with visual impairment from all over the country are referred to our clinic in order to benefit from the multidisciplinary approach and since, in most of them, an IED is the cause for the impaired vision, the family may undergo genetic counseling and testing if interested.
Data were taken from medical charts of patients who underwent genetic counseling from January 2015 to July 2023 and included children with impaired vision due to cataract or adults who came for genetic testing due to BPC in the family.
Details extracted from medical charts included demographic data, cataract characteristics, associated ocular and systemic conditions as well as genetic tests results. Information regarding the genetic analyses performed including molecular tests used, was also retrieved from medical files. The pathogenicity of each variant was determined by the lab [Invitae or Hadassah genetic lab] according to ACMG 2015 guidelines [11, 12]. Only pathogenic or likely pathogenic gene variants were considered in our study.
Results
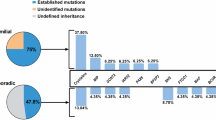
Our study included 47 patients (27 female and 20 male) from 22 unrelated families. Eighteen families were of Jewish origin (12/18 were Ashkenazi, followed by incidence by Morocco and Kurdistan Jewish, Table 1) and four were of Arab Muslim origin. Age at genetic testing ranged from 3 months to 43 years.
Genetic testing: Blood samples were collected for genetic testing from index patients. DNA was extracted using a DNA extraction kit (invitrogen. Molecular tests performed included NGS (i.e., whole exome sequencing and gene panel), Sanger sequencing, chromosomal microarray (CMA) and Multiplex Ligation-dependent Probe Amplification (MLPA), depending on patients’ preference and/or entity covering for genetic tests costs (HMO or private insurance). Blood samples from related family members were collected following identification of a causing pathogenic/likely pathogenic variant in the index patient. No instances of incomplete penetrance were identified.
A list containing the genes included in the panel-NGS based test associated with congenital cataract is provided as Supplementary material. Sanger sequencing was used for verification of variants as well as for segregation analysis.
Causative gene variants were identified in 18/22 families, including three novel and 15 known variants. Table 1 shows the gene variants spectrum found among our patients. Inheritance pattern was autosomal dominant (AD) in 13 out of 18 families, followed by X-linked dominant pattern in 3 of 18 families and autosomal recessive (AR) in two families.
Identified gene variants were mainly in crystalline genes (6/18 families; CRYBA1, CRYBB1, CRYBB2, and CRYGD genes) and in transcription factor genes (PAX6 and BCOR) in another 7/18 families (Table 2). Notably, 11 families performed genetic testing seeking pregnancy planning, of whom 7 had a causing gene identified and proceeded to pre-natal planning (Table 1).
The phenotypic characteristics of cataracts varied among studied patients. Mild anterior polar opacities, with minimal visual axis obstruction was seen in four families with PAX6 variants with only one patient requiring surgery at the age of 8 years (FAM22).
Progressive developmental nuclear cataract with two different identified genes, CRYBB1 (FAM12) and CYP27A1 (FAM17) caused moderate reduction in vision in two families (2 children each) needing surgery between four and seven years of age.
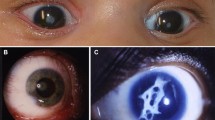
Dense lens opacities occluding visual axis that required early surgery (six months or earlier) were seen in seven families, two of which (FAM3 and FAM14) had a bilateral persistent fetal vasculature (PFV) cataract with an identified BCOR variant. Two families had a crystalline lens variant (FAM11 and FAM21) and another two families had a variant in a connexin gene (FAM13 and FAM16) (Table 1).
Cataract was believed to be isolated in all four families with PAX6 variants as well as in one family with BCOR and another with CYP27A1 variants; the accompanying ocular or systemic components were connected to the cataract only following genetic testing in these families.
Glaucoma was diagnosed in five of the 22 families; three of those had a BCOR gene variant. (Table 1).
Discussion
Genetic counseling and testing became during the last years a significant clinical service for families affected by an inherited eye condition. Molecular testing are not considered anymore for “research purposes” but more an essential part of the ophthalmological service. Patients get to understand the origin, transmission paths and possibilities of prevention.
Our study highlights the importance of accessible genetic testing for families with BPC. Variant rate detection varied in our two different ethnic groups, reaching 100% (4/4) in Muslim families while among Jewish families it was 77.7% (14/18).
Variants in crystalline genes were prevalent among isolated BPC, representing a significant percentage of the families in our cohort (6 out of 18) consistent with the existing literature [9, 27, 29]. Moreover, transcription factor genes (specifically PAX6 and BCOR) were detected in a substantial proportion (7 out of 18) among our studied families, surpassing the prevalence reported in prior studies [30].
Dense nuclear cataracts were identified in association with crystalline genes (CRYBA1, CRYBB2) as previously described [30] but also with connexin and BCOR genes in our population.
The co-occurrence of BPC and glaucoma was observed in 5 out of 22 families: 3 families exhibited a BCOR gene variant, while one family presented the recently reported GJA3 gene variant (c.199G > T), that has been associated with the co-occurrence of congenital cataract and glaucoma [21].
Patients in whom a variant was identified in the PAX6 gene were referred for genetic testing due to anterior polar cataract with or without nystagmus, and no other apparent ocular pathology. Upon thorough ocular examination at our center, mild corectopia and foveal hypoplasia were observed. One patient (FAM22) needed cataract extraction at the age of 8 years due to progressive posterior capsular opacification in addition to anterior polar cataract. This is in accordance with the literature describing a highly variable phenotype in PAX6 variants[] [31,32,33].
Syndromic involvement was identified in 4/22 families (18%), of which 3/4 had a BCOR variant causing OFCD syndrome. Interestingly, 2/3 families (3 females) with a different BCOR gene variant identified, had PFV type of cataract, needing early surgery. In one of these families (FAM3) a girl was born with a bilateral cataract with PFV and no obvious systemic pathology noticed during the first years of age. Her mother had a unilateral cataract that was believed not to be inherited. Upon molecular testing a variant was identified in the BCOR gene in the girl and later, mosaicism was found in the mother. To the best of our knowledge, the association between BCOR gene and PFV has not been previously reported in the literature.
In the fourth family with a syndromic involvement two variants in the CYP27A1 gene were identified reaching the diagnosis of cerebrotendinous-xanthomatosis (CTX). This family was under follow up due to bilateral developmental cataract in 2/6 of their children, with a background of delayed development in these children and another 2 siblings. Referral for genetic testing was done several times by treating ophthalmologists but parents did not comply. They opted to perform molecular testing when their children were eight and six years of age, finally reaching a molecular diagnosis. Of note, the variant CYP27A1: c.845-1G > A identified in this family, is a founder mutation in Moroccan Jews [22, 31].
The late molecular diagnosis at 6-to-8 years of age, such as in FAM17, can be preoccupying since many bilateral cataracts in infancy might be associated with a systemic condition or syndrome that can affect the child’s development or life span such as CTX, which has an existing treatment (chenodeoxycholic acid) that can prevent occurrence or progression of clinical manifestations of the disease if administered early enough [34, 35]. This fact enhances the importance of genetic testing in children with bilateral cataracts.
In addition, knowing the molecular etiology of a hereditary ocular disease in the family may also allow pre-marital counseling in certain populations as well as prenatal diagnosis and preimplantation genetic testing (PGT) in couples willing to do it [36].
We report three novel variants (two in CRYBB2 gene and one in MIP gene) that were all categorized as “likely pathogenic” by the responsible molecular laboratory. In two among these families, segregation showed an affected parent.
Our study has limitations such as its retrospective nature. Identified gene variants did not undergo functional analysis, and in FAM9 (with a novel variant), parents did not complete segregation tests as requested.
In conclusion, combined molecular techniques showed a high detection rate of causative gene variants in our population. The confirmation of pathogenic variants can help in family counseling regarding recurrence risks and family planning, and, in some cases may aid in the diagnosis of a non-suspected syndromic type of pediatric cataract.
Our results suggest that BCOR gene variants might be associated with a PFV cataract. Further research is warranted to explore and establish any potential link between the BCOR gene and PFV cataract.
References
Gilbert C (2007) Changing challenges in the control of blindness in children, in Eye, vol. 21, no. 10, pp. 1338–1343, https://doi.org/10.1038/sj.eye.6702841
Yahalom C, Kochavi MM, Mechoulam H, Cohen E, Anteby I (2022) Prevalence and factors related to visual impairments in children with bilateral cataract following surgery and the potential need for education and rehabilitation services. J Vis Impair Blind 116(1):61–69. https://doi.org/10.1177/0145482X211073588
Wu X, Long E, Lin H, Liu Y (2015) Prevalence and epidemiological characteristics of congenital cataract: a systematic review and meta-analysis. Sci. Rep 6(October):1–10, 2016, https://doi.org/10.1038/srep28564
Rahi JS, Dezateux C (2001) Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Investig Ophthalmol Vis Sci 42(7):1444–1448
Messina-Baas O, Cuevas-Covarrubias SA (2017) Inherited congenital cataract: a guide to suspect the genetic etiology in the cataract genesis. Mol Syndromol 8(2):58–78. https://doi.org/10.1159/000455752
Online Mendelian Inheritance in Man, OMIM® McKusick-Nathans Institute of Genetic Medicine. Baltimore, MD: Johns Hopkins University, 2018, [Online]. Available: https://omim.org/
Berry V, Georgiou M, Fujinami K, Quinlan R, Moore A, Michaelides M (2020) Inherited cataracts: molecular genetics, clinical features, disease mechanisms and novel therapeutic approaches. Br J Ophthalmol 104(10):1331–1337. https://doi.org/10.1136/bjophthalmol-2019-315282
Francis PJ, Moore AT (2004) Genetics of childhood cataract. Curr Opin Ophthalmol 15(1):10–15. https://doi.org/10.1097/00055735-200402000-00003
Shiels A, Bennett TM, Hejtmancik JF (2010) Cat-Map: putting cataract on the map. Mol Vis 16(August):2007–2015
Gillespie RL et al (2014) Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology 121(11):2124–2137. https://doi.org/10.1016/j.ophtha.2014.06.006
Rips J et al (2024) Unbiased phenotype and genotype matching maximizes gene discovery and diagnostic yield. Genet Med 26(4):101068. https://doi.org/10.1016/j.gim.2024.101068
Richards S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Martha A, Strong LC, Ferrell RE, Saunders GF (1995) Three novel h i r i d i a mutations in the human. vol. 4449
Hu Q, Mai J, Xiang Q, Zhou B, Liu S, Wang J (2022) A novel deletion mutation in the BCOR gene is associated with oculo-facio-cardio-dental syndrome: a case report. BMC Pediatr 22(1):1–10. https://doi.org/10.1186/s12887-022-03148-x
Santhiya ST et al (2002) Novel mutations in the γ-crystallin genes cause autosomal dominant congenital cataracts [6]. J Med Genet 39(5):352–358. https://doi.org/10.1136/jmg.39.5.352
Hilton E et al (2009) BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. Eur J Hum Genet 17(10):1325–1335. https://doi.org/10.1038/ejhg.2009.52
Kannabiran C et al (1998) Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol Vis 4(October):21
Reis LM et al (2013) Whole exome sequencing in dominant cataract identifies a new causative factor, CRYBA2, and a variety of novel alleles in known genes. Hum Genet 132(7):761–770. https://doi.org/10.1007/s00439-013-1289-0
Mohebi M, Akbari A, Babaei N, Sadeghi A, Heidari M (2016) Identification of a De novo 3 bp deletion in CRYBA1/A3 gene in autosomal dominant congenital cataract. Acta Med Iran 54(12):778–783
Cohen D et al (2007) Homozygous CRYBB1 deletion mutation underlies autosomal recessive congenital cataract. Investig Ophthalmol Vis Sci 48(5):2208–2213. https://doi.org/10.1167/iovs.06-1019
Brunetti-pierri N et al (2009) NIH public access. 40(12):1466–1471, https://doi.org/10.1038/ng.279.Recurrent
Ng D et al (2004) Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet 36(4):411–416. https://doi.org/10.1038/ng1321
Boese EA et al (2023) GJA3 genetic variation and autosomal dominant congenital cataracts and glaucoma following cataract surgery. JAMA Ophthalmol 141(9):872–879. https://doi.org/10.1001/jamaophthalmol.2023.3535
Leitersdorf E et al (1993) Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in jews of Moroccan origin. J Clin Invest 91(6):2488–2496. https://doi.org/10.1172/JCI116484
Reshef A, Meiner V, Berginer VM, Leitersdorf E (1994) Molecular genetics of cerebrotendinous xanthomatosis in jews of north African origin. J Lipid Res 35(3):478–483. https://doi.org/10.1016/s0022-2275(20)41198-8
Hanson I et al (1999) Missense mutations in the most ancient residues of the PAX6 paired domain underlie a spectrum of human congenital eye malformations. Hum Mol Genet 8(2):165–172. https://doi.org/10.1093/hmg/8.2.165
Souzeau E et al (2018) PAX6 molecular analysis and genotype-phenotype correlations in families with aniridia from Australasia and Southeast Asia. Mol Vis 24(January):261–273
Redeker EJW, de Visser ASH, Bergen AAB, Mannens MMAM (2007) Multiplex ligation-dependent probe amplification (MLPA) enhances the molecular diagnosis of aniridia and related disorders. Mol Vis 14(October):836–840.
Kumar M, Kaur P, Kumar M, Khokhar S, Dada R (2013) Molecular and structural analysis of genetic variations in congenital cataract. Mol Vis 19(November):2436–2450.
Rechsteiner D et al (2021) Genetic analysis in a Swiss cohort of bilateral congenital cataract. JAMA Ophthalmol 139(7):691–700. https://doi.org/10.1001/jamaophthalmol.2021.0385
Yahalom C et al (2018) Mild aniridia phenotype: an under-recognized diagnosis of a severe inherited ocular disease. Graefe’s Arch Clin Exp Ophthalmol 256(11):2157–2164. https://doi.org/10.1007/s00417-018-4119-1
Goolam S et al (2017) Familial congenital cataract, coloboma, and nystagmus phenotype with variable expression caused by mutation in PAX6 in a south african family. Mol Vis 24(July):407–413
Dansault A et al (2007) Three new PAX6 mutations including one causing an unusual ophthalmic phenotype associated with neurodevelopmental abnormalities, Mol. Vis, vol. 13, no. March, pp. 511–523
Berginer VM, Abeliovich D (1981) Genetics of cerebrotendinous xanthomatosis (CTX): an autosomal recessive trait with high gene frequency in sephardim of Moroccan origin. Am J Med Genet 10(2):151–157. https://doi.org/10.1002/ajmg.1320100209
Salen G, Steiner RD (2017) Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX). J Inherit Metab Dis 40(6):771–781. https://doi.org/10.1007/s10545-017-0093-8
Yahalom C et al (2018) Preimplantation genetic diagnosis as a strategy to prevent having a child born with an heritable eye disease. Ophthalmic Genet. https://doi.org/10.1080/13816810.2018.1474368
Funding
Open access funding provided by Hebrew University of Jerusalem.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any prospective studies with human participants or animals performed by any of the authors.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yahalom, C., Anteby, I., Hendler, K. et al. Genetics of bilateral pediatric cataract in the Israeli and Palestinian populations. Graefes Arch Clin Exp Ophthalmol 262, 3385–3391 (2024). https://doi.org/10.1007/s00417-024-06546-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-024-06546-2