Abstract
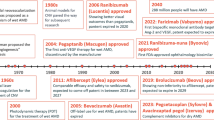
Intravitreal anti–vascular endothelial growth factor (VEGF) therapy is the standard of care for diabetic macular edema (DME) and neovascular age-related macular degeneration (nAMD); however, vision gains and anatomical improvements are not sustained over longer periods of treatment, suggesting other relevant targets may be needed to optimize treatments. Additionally, frequent intravitreal injections can prove a burden for patients and caregivers. Angiopoietin-2 (Ang-2) has been explored as an additional therapeutic target, due to the involvement of Ang-2 in DME and nAMD pathogenesis. Recent evidence supports the hypothesis that targeting both VEGF and Ang-2 may improve clinical outcomes in DME and nAMD compared with targeting VEGF alone by enhancing vascular stability, resulting in reduced macular leakage, prevention of neovascularization, and diminished inflammation. Faricimab, a novel bispecific antibody that targets VEGF-A and Ang-2, has been evaluated in clinical trials for DME (YOSEMITE/RHINE) and nAMD (TENAYA/LUCERNE). These trials evaluated faricimab against the anti-VEGFA/B and anti–placental growth factor fusion protein aflibercept, both administered by intravitreal injection. In addition to faricimab efficacy, safety, and pharmacokinetics, durability was evaluated during the trials using a treat-and-extend regimen. At 1 year, faricimab demonstrated non-inferior vision gains versus aflibercept across YOSEMITE/RHINE and TENAYA/LUCERNE. In YOSEMITE/RHINE, faricimab improved anatomic parameters versus aflibercept. Reduction of central subfield thickness (CST), and absence of both DME and intraretinal fluid were greater in faricimab- versus aflibercept-treated eyes. In TENAYA/LUCERNE, CST reductions were greater for faricimab than aflibercept at the end of the head-to-head phase (0–12 weeks), and were comparable with aflibercept at year 1, but with less frequent dosing. CST and vision gains were maintained during year 2 of both YOSEMITE/RHINE and TENAYA/LUCERNE. These findings suggest that dual Ang-2/VEGF-A pathway inhibition may result in greater disease control versus anti-VEGF alone, potentially addressing the unmet needs and reducing treatment burden, and improving real-world outcomes and compliance in retinal vascular diseases. Long-term extension studies (RHONE-X, AVONELLE-X) are ongoing. Current evidence suggests that dual inhibition with faricimab heralds the beginning of multitargeted treatment strategies inhibiting multiple, independent components of retinal pathology, with faricimab providing opportunities to reduce treatment burden and improve outcomes compared with anti-VEGF monotherapy.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intravitreal anti–vascular endothelial growth factor (VEGF) monotherapy is the standard of care for diabetic macular edema (DME) and neovascular age-related macular degeneration (nAMD) [1,2,3]. However, in DME, the Diabetic Retinopathy Clinical Research Network Protocol I and Protocol T comparative effectiveness studies found that after 2 years, vision stopped improving and vision gains were not sustained in large proportions of patients [4, 5]. Many patients also had persistent retinal thickening after 2 years [4, 5]. In trials of monthly ranibizumab in DME, up to 10% of eyes still progressed from early disease (i.e., non-proliferative diabetic retinopathy [NPDR]) to more severe disease (i.e., proliferative diabetic retinopathy [PDR]) after 2 years [6]. In the Protocol W trial of the anti–VEGF-A and anti–placental growth factor fusion protein aflibercept in DME, 4-year cumulative probability of developing PDR was 33.9% in the aflibercept arm versus 56.9% in the sham arm; additionally, 4-year cumulative incidence of center-involving DME with vision loss was 11.3% in the aflibercept group and 19.1% in the sham group [7]. This progression may reflect the complex and multifactorial pathophysiology of diabetic retinopathy (DR)/DME; therefore, novel treatments targeting other disease mechanisms beyond VEGF inhibition should be explored [6, 8].
In patients with nAMD, visual acuity (VA) gains observed during the first 2 years of designated anti-VEGF therapy were not maintained at 5 years in the Comparison of Age-related macular degeneration Treatment Trials follow-up study [9, 10]. Similarly, in HORIZON, an open-label extension study of the anti–VEGF-A antibody ranibizumab [11], incremental declines from initial VA gains were observed after 2 years. With aflibercept, in the VIEW studies, mean 1-year VA gains were broadly maintained but with small losses at years 2 and 4, and anatomic deterioration was reported [12, 13]. Furthermore, in the SEVEN-UP study, although approximately one-third of patients treated with ranibizumab had a good visual outcome after 7 years, one-third had a best-corrected VA (BCVA) of 20/200 or worse [14].
Analyses of real-world clinical practice data have shown that many patients do not achieve vision gains equivalent to those reported in clinical trials, and that many receive fewer anti-VEGF injections than in clinical trials [15,16,17,18,19,20,21,22]. The frequent intravitreal injections and close monitoring required to achieve optimal outcomes with intravitreal anti-VEGF monotherapy are a burden for patients and their caregivers [16, 20], and several risk factors for suboptimal treatment outcomes have been reported, including poor baseline VA, sociodemographic factors, older age, and co-existing health issues [18, 23, 24]. DME and nAMD have a multifactorial pathogenesis, including the influence of inflammatory mediators and growth factors, meaning that VEGF inhibition alone cannot address all aspects of each disease [8, 25].
As such, there remains a significant opportunity for improvement in treatment outcomes for patients with retinal vascular diseases, and a need for additional therapeutic strategies to reduce treatment burden and optimize vision outcomes for patients with DME and nAMD.
This review examines the rationale for targeting two pathways, angiopoietin-2 (Ang-2) in addition to VEGF, for treatment of DME and nAMD. We assess the results from recent clinical trials in patients with DME and nAMD that evaluated the efficacy, safety, pharmacokinetics, and durability of dual VEGF-A and Ang-2 inhibition with faricimab, a novel bispecific antibody directed against these two molecules.
The Ang/Tie pathway in vascular stability and remodeling
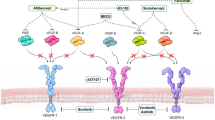
The Ang/tyrosine kinase with immunoglobulin-like and epidermal growth factor homology domains (Tie) pathway is essential for regulating vascular stability, angiogenesis, vascular permeability, and inflammation (Fig. 1) [26,27,28]. In healthy adult retinas with quiescent blood vessels, angiopoietin-1 (Ang-1) binds to and activates the Tie2 receptor, promoting vascular stability by stimulating endothelial cell survival and endothelial cell junction stability [26, 27]. In disease states, hypoxia and inflammation trigger an angiogenic switch that enhances production of Ang-2, which outcompetes (overwhelms) Ang-1 for Tie2 receptor occupancy [26, 27]. This sequence of events induces vascular permeability and instability through pericyte drop-out, leukocyte recruitment and adhesion, and weakening of endothelial cell junctions [27].
Simplified overview of the contribution of Ang/Tie2 and VEGF signaling to the regulation of vascular homeostasis. During the switch from stable vasculature to angiogenesis, Ang-1 levels are unchanged, but Ang-2 production increases and Ang-2 outcompetes Ang-1 for binding to Tie2. Ang-1 angiopoietin-1, Ang-2 angiopoietin-2, Tie2 tyrosine kinase with immunoglobulin-like and epidermal growth factor homology domains-2, VEGF vascular endothelial growth factor
Tie2 is a tyrosine kinase receptor expressed primarily on the cell surface of endothelial cells and some hematopoietic cells [27, 29,30,31], and it activates the phosphatidylinositol 3-kinase-protein kinase B pathway [27, 29,30,31]. Ang-2 is expressed by endothelial and extravascular cells, and is stored in intracellular Weibel-Palade bodies, where it is generally present at low levels except for in the microvascular endothelium [32] (the endothelium most affected in DR and AMD). Ang-2 is localized to active sites of angiogenesis/vascular remodeling and is released from Weibel-Palade bodies in response to thrombin or histamine [32, 33]. The role of Ang-2 in angiogenesis and vascular remodeling is further supported by studies of manipulation of Ang-2 expression in mouse models [34,35,36]. Although Ang-2 is not required for retinal development in the embryo, it is needed shortly after birth [37, 38]. Studies in animal models of disease have shown that Ang-2 collaborates with VEGF, alongside VEGF-independent effects, to activate pathological angiogenesis and promote vascular instability [39].
Vascular instability, VEGF, and Ang-2 in DME and DR
DME and DR are multifactorial in origin; microangiopathy and neurodegeneration, together with the inter-related effects of chronic hyperglycemia, hypoxia, low-grade inflammation, and proinflammatory cytokine release, trigger a number of changes, including pericyte loss, endothelial cell loss, capillary basal lamina thickening, retinal ischemia, and the breakdown of the blood-retina barrier [40,41,42]. These changes lead to the formation of microaneurysms, intraretinal microvascular abnormalities that are characteristic of NPDR, and to retinal neovascularization and vitreous hemorrhages, which are indicative of PDR [2, 43, 44]. Furthermore, as a result of the blood-retina barrier breakdown, localized fluid imbalances can lead to macular edema and thickening of the central fovea characteristic of center-involving DME [2, 43, 45,46,47].
Several processes that are central to the pathogenesis of DME, such as vessel instability through pericyte loss, loss of endothelial tight junctions, increased vascular permeability, and ischemia, can be experimentally manipulated in animal models, and are influenced by Ang-2 activity [27, 43, 47,48,49]. These features of DME pathogenesis are also associated with disease states, including inflammation, obesity, infectious disease (including complications such as sepsis), cancer, and cardiovascular disease. Ang-2 activity has also been shown to be involved in vascular instability in these conditions [50].
In a mouse ischemic retinopathy model, Ang-2 expression was increased in retinal endothelial cells and was necessary for retinal neovascularization [37, 51]. A 30-fold upregulation of Ang-2 was observed in the retinas of diabetic rats prior to the onset of pericyte loss, and intravitreal injection of Ang-2 induced dose-dependent pericyte loss without affecting vessel formation in normal rats [52]. In the same report, heterozygous Ang-2 deficiency in mice prevented diabetes-induced pericyte loss and reduced the number of acellular capillary segments [52]. Furthermore, inhibiting the activity of vascular endothelial protein tyrosine phosphate (VE-PTP), a negative regulator of Tie2, has been shown to reduce ischemia-induced retinal neovascularization in mice [53]. These findings suggest that Ang-2/Tie2 plays a role in retinal neovascularization in the ischemic retina, and that inhibition of Ang-2 could be an effective approach to reduce angiogenesis in ischemic retinal diseases.
In addition to animal model data, findings in patients with ischemic retinal disease also support a role for Ang-2/Tie2 and VEGF pathways in retinal neovascularization. In patients with DR and retinal vein occlusion, vitrectomized epiretinal membranes showed upregulation of Ang-2, Tie2, and VEGF, but not Ang-1, proteins [54]. Additionally, intraocular VEGF is upregulated in the vitreous of patients with PDR and DME [55,56,57]. Other studies have corroborated increased Ang-2 and VEGF in the vitreous of patients with DR, as well as found increased concentrations of matrix metalloproteases-2 and -9, erythropoietin, and transforming growth factor-β [58, 59]. However, while VEGF-induced vascular leakage plays a key role in DME, it is not the whole story. For instance, alterations in the intraocular cytokine milieu after anti-VEGF therapy (with bevacizumab) suggest the presence of compensatory mechanisms involving other cytokines and growth factors in response to VEGF inhibition [60]. Connective tissue growth factor plays a role in DR, suggesting that treatments targeting the fibrotic pathway may be required in DR [61]. Macular edema resulting from vascular leakage can cause irreversible vision loss; patients may therefore benefit from treatment that targets early vascular destabilization and remodeling in NPDR, before progression to PDR and/or DME [62]. In summary, findings from preclinical studies and from patients with DR and DME suggest that Ang-2 is a potential target for evaluation in this context due to its influence on vascular stability, independent from VEGF.
Vascular instability, VEGF, and Ang-2 in nAMD
In nAMD, pathological changes occur within the choroid-retinal pigment epithelium (RPE) complex. Thickening and disruption of Bruch’s membrane and the presence of sub-RPE deposits and drusen contribute to oxidative stress in the RPE due to N-retinylidene-N-retinylethanolamine (A2E) accumulation, and chronic inflammation via the complement cascade and membrane attack complex, thereby causing loss of the choriocapillaris [63,64,65,66]. Disruption of the cross-talk between the RPE and choriocapillaris endothelial cells results in choriocapillaris loss [63,64,65,66,67,68]. In the absence of the choriocapillaris, localized macular hypoxia, oxidative stress, and inflammation upregulate VEGF, stimulating the growth of new blood vessels arising from the choroid (type 1 and 2 macular neovascularization [MNV]) or the retina (type 3 MNV) [65]. Nevertheless, the combination of immature blood vessel formation, vessel instability, and permeability (through loss of endothelial tight junctions pericytes), breakdown of the outer blood-retinal barrier, and inflammation are central to the pathogenesis of nAMD, suggesting a possible role for Ang-2 inhibition in its treatment [69, 70].
Preclinical studies have shown that Ang/Tie2 plays an important role in the choriocapillaris during its development and maintenance in the healthy adult eye, as well as in models of choroidal neovascularization (CNV) disease [53, 70, 71]. A high Ang-2/VEGF ratio promotes regression of neovascularization, whereas elevation of both Ang-2 and VEGF leads to increased neovascularization, suggesting that simultaneous inhibition of Ang-2 and VEGF could prevent further choroidal vasculature remodeling [45]. Findings in human tissue and isolated human cells have additionally highlighted the role of Ang/Tie2 in nAMD. In aqueous humor samples from patient eyes with nAMD, Ang-2 levels were elevated and correlated with disease severity [72]. It has also been observed in clinical trials of anti-VEGF monotherapy that when treatment leads to CNV regression in nAMD, patients may be left with macular atrophy and fibrosis at the site of regression; however, if type 1 MNV is appropriately treated, there may be protection from macular atrophy and fibrosis [73, 74]. Consequently, there is a need in nAMD for therapies that control and stabilize actively growing choroidal vasculature. In summary, similar to DME, findings from preclinical studies and from patients with nAMD suggest that Ang-2 could be a potential target for evaluation in this context due to its influence on vascular stability, independent from VEGF.
Dual Ang-2 and VEGF inhibition in retinal vascular diseases
The synergistic relationship between Ang-2 and VEGF in the promotion of vascular leakage and inflammation supports the concept of dual pathway inhibition to promote vascular stability and improve outcomes beyond anti-VEGF therapies for patients with retinal vascular diseases. Studies in tumor models have suggested a compensatory relationship between Ang-2 and VEGF in pathologic angiogenesis, whereby inhibition of one molecule results in a shift to upregulate the other [75]. This may explain why long-term efficacy is difficult to maintain with VEGF inhibition alone, and provides further rationale for targeting Ang-2 and VEGF simultaneously [9, 14]. In a dual inducible mouse model in which both Ang-2 and VEGF could be overexpressed, Oshima et al. [39] showed that Ang-2 sensitized retina vessels to the angiogenic effects of VEGF. No data are available that show VEGF elevation can sensitize retinal vasculature to Ang-2 activity in a similar way. It is reasonable to expect that durable suppression of Ang-2 can limit the biologic activity of VEGF, reducing neovascularization and vessel permeability. This was demonstrated in transgenic mice with inducible Ang-2 expression during experimental hypoxia (i.e., when VEGF levels are high), when Ang-2 expression promoted retinal neovascularization [76]. Following resolution of hypoxia (i.e., when VEGF levels are low), Ang-2 expression promotes regression of retinal neovascularization. These findings support the notion that Ang-2 induces vascular stability after an angiogenic switch, in a VEGF-independent manner [26,27,28]. The study also showed that the context of VEGF elevation was important for the effect of Ang-2, as vasculature in the deep capillary bed was more sensitive to Ang-2 elevation during early embryonic development, with no effect on the superficial capillary bed [76]. At later stages of embryonic development, the morphology of the deep capillary bed was similar to that of wild-type mice.
In the eye, dual Ang-2/VEGF pathway inhibition in a mouse model of retinal angiomatous proliferation, using aflibercept in combination with an inhibitor (AKB-9778) of the negative regulator of Tie, VE-PTP, showed an additive effect on reducing subretinal neovascularization [53]. Similarly, in a mouse model of spontaneous CNV [77], dual VEGF-A/Ang-2 inhibition yielded reductions from baseline in CNV lesion leakage area versus either anti–VEGF-A or anti–Ang-2 monotherapy [77, 78]. Reductions in retinal inflammation adjacent to CNV lesions and photoreceptor apoptosis were also observed with dual VEGF-A/Ang-2 inhibition in this study. Dual VEGF/Ang-2 inhibition also decreased retinal leukocyte infiltration and aqueous humor inflammatory cells in a mouse model of endotoxin-induced uveitis, whereas Ang-2 or VEGF inhibition alone showed no effect [79].
Combination therapy with anti-VEGF and anti–Ang-2 agents has been evaluated in patients with retinal vascular disease. A phase 2 study in DME, RUBY, found that intravitreal aflibercept combined with the anti–Ang-2 antibody, nesvacumab, achieved similar BCVA gains to aflibercept monotherapy over 36 weeks; however, mean reductions in central subfield thickness (CST) and rates of CST resolution, as well as other anatomic parameters, were significantly higher with aflibercept/nesvacumab combination therapy [80]. ONYX, a phase 2 superiority study in nAMD, similarly showed that mean 36-week vision gains with aflibercept plus nesvacumab were not significantly different from those with aflibercept monotherapy, although trends toward greater mean CST reduction with combination therapy versus anti-VEGF monotherapy were observed [81]. Although the findings of RUBY and ONYX showed anatomic benefits with combined aflibercept and nesvacumab therapy, vision outcomes were comparable to aflibercept monotherapy; therefore, further development of this combination therapy for nAMD and DME was not supported [82]. Nevertheless, the results of these studies support the hypothesis that the addition of Ang-2 pathway inhibition may promote vascular stability and improve anatomic outcomes and durability beyond VEGF inhibition alone, based on the VEGF-independent activities of Ang-2.
Faricimab: a novel bispecific antibody designed for intraocular use
The bispecific antibody faricimab (F. Hoffmann-La Roche Ltd., Basel, Switzerland) was developed on the premise that neutralization of both VEGF-A and Ang-2 may synergistically promote vascular stability in retinal vascular diseases, and improve outcomes for patients [77, 83]. Faricimab was designed using CrossMAb technology (F. Hoffmann-La Roche Ltd.) and is based on a human immunoglobulin G1 framework, with two fragment antigen-binding arms that bind VEGF-A and Ang-2 with high specificity and potency [77, 78, 83, 84]. The fragment crystallizable (Fc) region of faricimab is engineered to allow for faster systemic clearance, reduced systemic exposure, and reduced inflammatory potential. Removal of the Fc gamma receptor binding site has the potential to eradicate antibody-dependent cytotoxicity, antibody-dependent cell phagocytosis, and complement-dependent cytotoxicity. Deletion of the neonatal Fc receptor binding site prevents intracellular immunoglobulin G recycling, thereby reducing the systemic half-life of faricimab treatment compared with wild-type immunoglobulin G, and decreasing the likelihood of potential systemic toxicity [77]. In a laser-induced CNV model in non-human primates, intravitreal faricimab elicited a significantly greater reduction in CNV (as assessed by scored fluorescent angiograms) and vessel leakage compared with anti–VEGF-A and anti–Ang-2 monotherapy [77, 78].
In a phase 1 trial in patients with nAMD and subfoveal CNV refractory to prior anti-VEGF therapy (NCT01941082), faricimab (dosed from 0.5 to 6.0 mg) was well tolerated with an acceptable safety profile, and there was preliminary evidence of improved BCVA and reductions in CST (Table 1) [85].
Faricimab for the treatment of DME
BOULEVARD, a phase 2, randomized, controlled, 36-week study, evaluated the safety and efficacy of faricimab in 229 patients (both treatment-naïve and previously treated) aged ≥ 18 years with center-involved DME (BCVA of 73–24 Early Treatment of Diabetic Retinopathy study [ETDRS] letters; CST ≥ 325 µm). Patients received intravitreal faricimab 6.0 mg every 4 weeks (Q4W), faricimab 1.5 mg Q4W, or ranibizumab 0.3 mg (US Food and Drug Administration–approved DME dose) Q4W up to week 20 [91]. Faricimab showed statistically significant BCVA gains achieving superiority at week 24 from baseline with faricimab 6.0 mg dosed monthly, compared with monthly ranibizumab in treatment-naïve patients (p = 0.03). Anatomic outcomes were also improved with faricimab versus ranibizumab. No new or unexpected safety signals were reported (Table 1) [91].
The phase 3, multicenter, randomized, active comparator-controlled, double-masked, 100-week, non-inferiority trials YOSEMITE (NCT03622580) and RHINE (NCT03622593) were identically designed to assess the safety, efficacy, and durability of faricimab in anti-VEGF treatment-naïve and previously treated patients with DME (Table 1) [92, 93]. Patients aged ≥ 18 years with macular thickening secondary to DME involving the center of the fovea (CST ≥ 325 µm) and a BCVA of 25–73 ETDRS letters (20/320–20/40 approximate Snellen equivalent) were eligible for inclusion in the studies. In total, 1891 patients across 353 sites worldwide were randomized 1:1:1 to intravitreal faricimab 6.0 mg every 8 weeks (Q8W) after six initial Q4W doses; faricimab 6.0 mg according to a personalized treat-and-extend-based regimen (T&E) after four initial Q4W doses; or aflibercept consistent with its globally aligned posology, comprising five initial Q4W doses followed by Q8W injections through to week 96 [100, 101]. Patients in the faricimab T&E arm received faricimab 6.0 mg Q4W until they reached a CST < 325 µm, at or after week 12. Once achieved, treatment intervals were extended to Q8W, then could be maintained, extended by 4 weeks (up to every 16 weeks [Q16W]), or reduced by 4 or 8 weeks (to as low as Q4W) based on prespecified CST and BCVA criteria at active dosing visits. The T&E arm was designed to assess the durability of faricimab using a standardized method designed to replicate a T&E regimen as in routine clinical practice.
The primary endpoint of non-inferior 1-year vision gains with faricimab Q8W or T&E versus aflibercept Q8W was met in both YOSEMITE and RHINE. Adjusted mean BCVA change from baseline at the primary endpoint visits (averaged over weeks 48, 52, and 56) in the pooled DME population from YOSEMITE/RHINE was + 11.2 (95% confidence interval [CI], 10.5–12.0) ETDRS letters with faricimab Q8W, + 11.2 (95% CI, 10.4–11.9) with faricimab T&E, and + 10.5 (95% CI, 9.8–11.3) with aflibercept Q8W [102].
In YOSEMITE/RHINE, the faricimab arms achieved greater reductions in CST over 1 year of treatment compared with aflibercept [102]. Adjusted mean CST change from baseline at year 1 (averaged over weeks 48, 52, and 56; 95% CI) in the pooled DME population was –200.9 (–206.7 to –195.1) µm with faricimab Q8W, –192.4 (–198.1 to –186.6) µm with faricimab T&E, and –170.2 (–176.0 to –164.4) µm with aflibercept Q8W [102]. Greater reductions in CST with faricimab were also observed during the head-to-head phase, when all arms received the same number of doses (0–16 weeks) [102]. Through year 1, more faricimab-treated patients achieved absence of protocol-defined DME (CST < 325 µm) compared with aflibercept-treated patients (81%–89% with faricimab Q8W and 82%–85% with T&E versus 68%–74% with aflibercept Q8W, at weeks 48–56) [102]. In addition, more faricimab- versus aflibercept-treated patients achieved absence of intraretinal fluid through week 56 (41%–46% with faricimab Q8W and 33%–42% with T&E versus 22%–27% with aflibercept Q8W, at weeks 48–56) [102]. Rates of absence of subretinal fluid were ≥ 96% at week 52 and comparable across all three study arms [102]. The proportion of patients with rates of ≥ 2-step ETDRS Diabetic Retinopathy Severity Scale score improvement from baseline at week 52 was consistently > 40% across faricimab treatment arms (45.1% and 43.1% with faricimab Q8W and T&E, respectively), and was similar to those observed in the aflibercept Q8W arm (41.3%) [102]. Outcomes were achieved with 51.9% of patients in the faricimab T&E arms on Q16W dosing at the week 52 visit, and in 72.4% on every-12-week (Q12W) dosing or longer [102].
Overall, faricimab was well tolerated with an acceptable safety profile that was comparable to that of aflibercept [93]. Incidence of ocular events in the study eye was similar between patients receiving faricimab Q8W (37.3%), faricimab T&E (35.6%), or aflibercept Q8W (34.4%). Serious ocular events were also comparable between patients receiving faricimab Q8W (2.4%), faricimab T&E (3.0%), or aflibercept Q8W (1.3%). In both trials, rates of intraocular inflammation were low (1.3%, 1.4%, and 0.6% with faricimab Q8W, faricimab T&E, and aflibercept Q8W, respectively). Investigators reported no cases of retinal vasculitis or occlusive retinal vasculitis.
To summarize, the 1-year efficacy results of YOSEMITE and RHINE demonstrated that faricimab Q8W or T&E resulted in non-inferior vision gains versus aflibercept Q8W, while demonstrating improved disease control, through improved anatomic outcomes and the potential for extended durability of up to Q16W. Recently published results including year 2 of faricimab treatment from YOSEMITE and RHINE show that vision gains, anatomical control and treatment durability were maintained through the second year of treatment [94]. Together, these findings support the hypothesis of an anatomical benefit from Ang-2 inhibition in DME and suggest that dual Ang-2/VEGF-A pathway inhibition may promote vascular stability beyond that achieved with VEGF inhibition alone. Additional data are included in the published primary results of the YOSEMITE and RHINE trials [92,93,94].
Faricimab for the treatment of nAMD
AVENUE was a phase 2, randomized, controlled, 36-week trial in 273 faricimab treatment-naïve patients aged ≥ 50 years with subfoveal CNV secondary to nAMD (BCVA 73–24 ETDRS letters [approximate Snellen equivalent 20/40–20/320]) (Table 1). It evaluated intravitreal faricimab 1.5 mg administered Q4W, faricimab 6.0 mg Q4W, faricimab 6.0 mg Q8W after four initial Q4W doses, faricimab 6.0 mg Q4W after three initial ranibizumab 0.5 mg Q4W doses, and ranibizumab 0.5 mg Q4W [86]. Patients who received either faricimab dose achieved and maintained BCVA and anatomic improvements, including CST, CNV area, and leakage, at a similar level to those achieved with ranibizumab [86]. A phase 2, randomized, controlled, 52-week trial, STAIRWAY, evaluated the efficacy and safety of extended dosing intervals with faricimab in 76 treatment-naïve patients aged ≥ 50 years with subfoveal or juxtafoveal CNV secondary to nAMD (BCVA and Snellen equivalents as in AVENUE) (Table 1). Intravitreal ranibizumab 0.5 mg administered Q4W was compared with faricimab 6.0 mg administered as four initial Q4W doses followed by dosing Q12W or Q16W through week 52 [87]. The BCVA gains at week 40 were comparable for faricimab Q12W or Q16W and ranibizumab, as were changes in CST and total lesion area [87]. Faricimab therefore demonstrated sustained efficacy at dosing intervals of up to Q16W. Faricimab was well tolerated, with an acceptable safety profile comparable to ranibizumab, and no new or unexpected safety events were identified in these phase 2 studies [86, 87].
The phase 3 TENAYA (NCT03823287) and LUCERNE (NCT03823300) trials were identically designed to evaluate the safety, efficacy, and durability of faricimab in treatment-naïve patients with nAMD (Table 1) [88, 89]. These were multicenter, randomized, active comparator-controlled, double-masked trials of 112 weeks’ duration. The trials included patients who were aged ≥ 50 years at enrollment, with a BCVA of 24–78 ETDRS letters (20/320–20/32 approximate Snellen equivalent), and either subfoveal CNV, or juxtafoveal or extrafoveal CNV with a subfoveal component. In total, 1329 patients with nAMD across 271 study sites worldwide were randomized 1:1 to aflibercept 2.0 mg Q8W after three initial Q4W doses, as per its globally aligned posology, or faricimab 6.0 mg [100, 101]. After four initial Q4W doses of faricimab, disease activity was determined based on CST and investigator-assessed BCVA and presence of macular hemorrhage. Patients with active disease at week 20 then received Q8W dosing through week 60, those with active disease at week 24 received Q12W dosing through week 60, and those with no active disease at weeks 20 and 24 were treated with faricimab at week 28 and remained on Q16W dosing through week 60. From week 60 to week 112, all patients in the faricimab arm were treated according to a T&E regimen, in which dosing intervals could be extended by 4 weeks, maintained, or reduced by 4 or 8 weeks from Q8W up to Q16W according to disease activity assessments.
The primary efficacy endpoint of non-inferiority in mean BCVA change from baseline at the primary endpoint visits (averaged over weeks 40, 44, and 48) with faricimab up to Q16W versus aflibercept Q8W was met in TENAYA and LUCERNE, with an adjusted mean gain from baseline of + 6.2 ETDRS letters (95% CI, 5.3–7.1) with faricimab and + 5.9 (95% CI, 5.0–6.7) with aflibercept in the pooled nAMD population [103]. At the primary endpoint visits, approximately 20% of patients gained ≥ 15 ETDRS letters from baseline with faricimab up to Q16W and with aflibercept Q8W, and 96% of patients across both treatment arms avoided losses of ≥ 15 ETDRS letters from baseline [104].
Anatomically, the adjusted mean changes in CST from baseline at the primary endpoint visits (averaged over weeks 40, 44, and 48) were comparable between faricimab up to Q16W and aflibercept Q8W, and were −137.0 µm (95% CI, −141.2 to −132.9 µm) and −130.1 µm (95% CI, −134.2 to −125.9), respectively [103]. However, greater reductions in CST with faricimab over aflibercept were observed during the head-to-head phase (0–12 weeks) [105]. These vision outcomes were achieved with approximately 45% of faricimab-treated patients in TENAYA/LUCERNE receiving Q16W dosing at week 48 [103].
In TENAYA and LUCERNE, faricimab was well tolerated and had an acceptable safety profile, with a low incidence of adverse events (AEs) leading to study treatment discontinuation [88]. Incidence of ocular AEs and serious ocular AEs through week 48 were generally similar between faricimab (ocular AEs, 38.3%; serious ocular AEs, 1.7%) and aflibercept (ocular AEs, 37.2%; serious ocular AEs, 2.0%). Rates of intraocular inflammation were low (2.0% for faricimab and 1.2% for aflibercept), and there were no investigator-reported events of retinal vasculitis or retinal occlusion associated with intraocular inflammation events in either study.
In summary, the 1-year efficacy results of TENAYA and LUCERNE demonstrated that faricimab up to Q16W offered non-inferior vision gains and improved anatomic outcomes versus aflibercept Q8W, supporting the hypothesis that dual Ang-2/VEGF-A pathway inhibition with faricimab may promote sustained efficacy for patients with nAMD. In TENAYA and LUCERNE, 1-year CST outcomes with faricimab were similar to those with aflibercept Q8W and were achieved with Q16W dosing in approximately 45% and with ≥ Q12W dosing in almost 80% of faricimab-treated patients. Year 2 results of faricimab treatment from TENAYA and LUCERNE show that vision gains, anatomical control, and treatment durability were maintained through the second year of treatment, with a greater proportion of patients achieving Q16W dosing versus year 1 [90]. Additional data are included in the published primary results of the TENAYA and LUCERNE trials [88,89,90].
Discussion
Clinical implications and conclusions
The introduction of anti-VEGF therapies revolutionized the management of retinal vascular disease. Real-world long-term outcomes with anti-VEGF monotherapy are improving, but they still fall below expectations. Possible approaches to improving VA and durability outcomes include increasing the dose, changing the drug delivery paradigm, and introducing a new mode of action. It is important to note that increased dosing of anti-VEGF agents has not led to improvements in clinical outcomes, particularly during the initial part of the study when dosing interval was matched with treatments [106,107,108,109]. Preclinical evidence suggests that dual Ang-2/VEGF inhibition may promote vascular stability and reduce the neovascularization and chronic inflammation compared with anti-VEGF inhibition alone [77,78,79].
Faricimab is a novel bispecific anti–Ang-2 and anti–VEGF-A antibody designed for intraocular use that has been evaluated in a phase 3 clinical trial program in retinal disease. In these studies, patients with DME or nAMD treated with faricimab demonstrated non-inferior vision gains, improved anatomical outcomes, and a similar safety profile versus aflibercept in the head-to-head phase, with ≥ 72% of patients in the faricimab T&E arms achieving Q12W dosing or longer at weeks 52 and 48 in YOSEMITE/RHINE and TENAYA/LUCERNE, respectively. The extended dosing intervals achieved with faricimab support the concept that Ang-2/VEGF-A–targeted therapies may address the unmet need for durable treatments that improve real-world outcomes and reduce the treatment burden compared with standard-of-care therapies for patients with retinal vascular diseases.
Results from studies assessing the correlation between anatomical outcomes and visual acuity are mixed. While some have shown that the absence of fluid is associated with visual improvements, others have demonstrated a poor correlation between the presence of fluid and visual outcomes, with vision loss not always associated with new fluid [110]. Further research is warranted to understand why the improvements in anatomical outcomes observed with faricimab in the phase 3 clinical trial program did not translate into improved vision outcomes versus aflibercept.
The promising results for patients with DME and nAMD suggest that faricimab may also be efficacious in other retinal vascular diseases. Phase 3 studies are under way in patients with macular edema secondary to central retinal or hemiretinal vein occlusion (COMINO; NCT04740931) [111], and in those with macular edema secondary to branch retinal vein occlusion (BALATON; NCT04740905) [112].
Results from the open-label extension study, RHONE-X (NCT04432831) (Table 1) [98], will inform the long-term safety, efficacy, and durability of faricimab in patients with DME. Similarly, the open-label extension study, AVONELLE-X (NCT04777201) (Table 1) [95], will provide further data on the safety, efficacy, and durability of faricimab in patients with nAMD. ELEVATUM (NCT05224102) (Table 1) is a phase 4 trial designed to improve understanding of faricimab in under-represented patients with DME, and the associated barriers that limit trial recruitment and retention in these populations [99]. SALWEEN (ISRCTN69073386) is a phase 4 study that will assess the efficacy, durability and safety of faricimab in polypoidal choroidal vasculopathy, a subtype of nAMD and a population under-represented in TENAYA/LUCERNE, in Asia (Table 1) [96]. Lastly, the phase 2b ALTIMETER (NCT04597918) (Table 1) biomarker hypothesis-generating study is exploring the associations between clinical endpoints, multimodal imaging assessments, and aqueous humor biomarker patterns in patients with DME treated with faricimab [97]. These studies may help demonstrate the benefits of Ang-2/VEGF-A co-inhibition in additional patient populations and over longer treatment periods.
Current evidence suggests that dual inhibition of Ang-2 and VEGF-A with faricimab may signal an important shift toward multitargeted treatment strategies for patients with DME, nAMD, and potentially other retinal vascular diseases, to improve outcomes versus anti-VEGF alone.
References
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS (2020a) Age-related macular degeneration Preferred Practice Pattern. Ophthalmol 127:P1–P65. https://doi.org/10.1016/j.ophtha.2019.09.024
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS (2020b) Diabetic retinopathy preferred practice pattern(R). Ophthalmol 127:P66–P145. https://doi.org/10.1016/j.ophtha.2019.09.025
Bro T, Derebecka M, Jorstad OK, Grzybowski A (2020) Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol 258:503–511. https://doi.org/10.1007/s00417-019-04569-8
Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117:1064–1077.e1035. https://doi.org/10.1016/j.ophtha.2010.02.031
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW, Diabetic Retinopathy Clinical Research Network (2016) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmol 123:1351–1359. https://doi.org/10.1016/j.ophtha.2016.02.022
Ip MS, Domalpally A, Sun JK, Ehrlich JS (2015) Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 122:367–374. https://doi.org/10.1016/j.ophtha.2014.08.048
Maturi RK, Glassman AR, Josic K, Baker CW, Gerstenblith AT, Jampol LM, Meleth A, Martin DF, Melia M, Punjabi OS, Rofagha S, Salehi-Had H, Stockdale CR, Sun JK, Network DR (2023) Four-year visual outcomes in the protocol w randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA 329:376–385. https://doi.org/10.1001/jama.2022.25029
Wang W, Lo ACY (2018) Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci 19:1816. https://doi.org/10.3390/ijms19061816
Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, Toth CA, Ferris FL 3rd, Fine SL (2016) Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmol 123:1751–1761. https://doi.org/10.1016/j.ophtha.2016.03.045
Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmol 119:1388–1398. https://doi.org/10.1016/j.ophtha.2012.03.053
Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, Tuomi L (2012) HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 119:1175–1183. https://doi.org/10.1016/j.ophtha.2011.12.016
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 121:193–201. https://doi.org/10.1016/j.ophtha.2013.08.011
Kaiser PK, Singer M, Tolentino M, Vitti R, Erickson K, Saroj N, Berliner AJ, Chu KW, Zhu X, Williams Liu Z, Clark WL (2017) Long-term safety and visual outcome of intravitreal aflibercept in neovascular age-related macular degeneration: VIEW 1 extension study. Ophthalmol Retina 1:304–313. https://doi.org/10.1016/j.oret.2017.01.004
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 120:2292–2299. https://doi.org/10.1016/j.ophtha.2013.03.046
Ciulla TA, Huang F, Westby K, Williams DF, Zaveri S, Patel SC (2018) Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the united states. Ophthalmol Retina 2:645–653. https://doi.org/10.1016/j.oret.2018.01.006
Ciulla TA, Pollack JS, Williams DF (2021) Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol 105:216–221. https://doi.org/10.1136/bjophthalmol-2020-315933
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, Hoyng CB, Hykin P, Staurenghi G, Heldner S, Bogumil T, Heah T, Sivaprasad S (2015) Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 99:220–226. https://doi.org/10.1136/bjophthalmol-2014-305327
Pearce I, Clemens A, Brent MH, Lu L, Gallego-Pinazo R, Minnella AM, Creuzot-Garcher C, Spital G, Sakamoto T, Dunger-Baldauf C, McAllister IL, all the Lsi (2020) Real-world outcomes with ranibizumab in branch retinal vein occlusion: The prospective, global LUMINOUS study. PLoS One 15:e0234739. https://doi.org/10.1371/journal.pone.0234739
Cantrell RA, Lum F, Chia Y, Morse LS, Rich WL 3rd, Salman CA, Willis JR (2020) Treatment patterns for diabetic macular edema: an Intelligent Research in Sight (IRIS®) Registry analysis. Ophthalmol 127:427–429. https://doi.org/10.1016/j.ophtha.2019.10.019
Ciulla T, Pollack JS, Williams DF (2021) Visual acuity outcomes and anti-VEGF therapy intensity in macular oedema due to retinal vein occlusion: a real-world analysis of 15 613 patient eyes. Br J Ophthalmol 105:1696–1704. https://doi.org/10.1136/bjophthalmol-2020-317337
Ciulla TA, Hussain RM, Pollack JS, Williams DF (2020) Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 Eyes. Ophthalmol Retina 4:19–30. https://doi.org/10.1016/j.oret.2019.05.017
Kiss S, Malangone-Monaco E, Wilson K, Varker H, Stetsovsky D, Smith D, Garmo V (2020) Real-world injection frequency and cost of ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration and diabetic macular edema. J Manag Care Spec Pharm 26:253–266. https://doi.org/10.18553/jmcp.2020.19245
Wubben TJ, Johnson MW, Anti VTISG (2019) Anti-vascular endothelial growth factor therapy for diabetic retinopathy: consequences of inadvertent treatment interruptions. Am J Ophthalmol 204:13–18. https://doi.org/10.1016/j.ajo.2019.03.005
Obeid A, Gao X, Ali FS, Talcott KE, Aderman CM, Hyman L, Ho AC, Hsu J (2018) Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmol 125:1386–1392. https://doi.org/10.1016/j.ophtha.2018.02.034
Flores R, Carneiro A, Vieira M, Tenreiro S, Seabra MC (2021) Age-related macular degeneration: pathophysiology, management, and future perspectives. Ophthalmol 244:495–511. https://doi.org/10.1159/000517520
Thurston G, Daly C (2012) The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med 2:a006550. https://doi.org/10.1101/cshperspect.a006650
Saharinen P, Eklund L, Alitalo K (2017) Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov 16:635–661. https://doi.org/10.1038/nrd.2016.278
Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE (2011) Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121:2278–2289. https://doi.org/10.1172/JCI46322
Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA (2000) Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res 87:603–607
Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY (2000) Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-Kinase/Akt signal transduction pathway. Circ Res 86:24–29. https://doi.org/10.1161/01.res.86.1.24
Koh GY (2013) Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med 19:31–39. https://doi.org/10.1016/j.molmed.2012.10.010
Mandriota SJ, Pepper MS (1998) Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 83:852–859. https://doi.org/10.1161/01.res.83.8.852
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150–4156. https://doi.org/10.1182/blood-2003-10-3685
Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG (2013) Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS ONE 8:e70459. https://doi.org/10.1371/journal.pone.0070459
Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12:235–239. https://doi.org/10.1038/nm1351
Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P (2015) Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat Commun 6:5962. https://doi.org/10.1038/ncomms6962
Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA (2002) Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol 192:182–187. https://doi.org/10.1002/jcp.10128
Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD (2002) Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 3:411–423. https://doi.org/10.1016/s1534-5807(02)00217-4
Oshima Y, Deering T, Oshima S, Nambu H, Reddy PS, Kaleko M, Connelly S, Hackett SF, Campochiaro PA (2004) Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol 199:412–417. https://doi.org/10.1002/jcp.10442
Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M (2002) Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 51:2241–2248. https://doi.org/10.2337/diabetes.51.7.2241
Romero-Aroca P (2010) Targeting the pathophysiology of diabetic macular edema. Diabetes Care 33:2484–2485. https://doi.org/10.2337/dc10-1580
Beltramo E, Porta M (2013) Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem 20:3218–3225. https://doi.org/10.2174/09298673113209990022
Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R (2016) Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res 2016:2156273. https://doi.org/10.1155/2016/2156273
Early Treatment Diabetic Retinopathy Study Research Group (1991) Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmol 98:823–833
Campochiaro PA (2015) Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res 49:67–81. https://doi.org/10.1016/j.preteyeres.2015.06.002
Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gelize E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F (2018) Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res 63:20–68. https://doi.org/10.1016/j.preteyeres.2017.10.006
Agarwal D, Gelman R, Prospero Ponce C, Stevenson W, Christoforidis JB (2015) The vitreomacular interface in diabetic retinopathy. J Ophthalmol 2015:392983. https://doi.org/10.1155/2015/392983
Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS (2004) Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev 18:1060–1071. https://doi.org/10.1101/gad.1189704
Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL (2003) Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93:1074–1081. https://doi.org/10.1161/01.RES.0000102937.50486.1B
Akwii RG, Sajib MS, Zahra FT, Mikelis CM (2019) Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells 8:471. https://doi.org/10.3390/cells8050471
Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA (2000) Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol 184:275–284. https://doi.org/10.1002/1097-4652(200009)184:3%3c275::AID-JCP1%3e3.0.CO;2-7
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53:1104–1110. https://doi.org/10.2337/diabetes.53.4.1104
Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K, Kojima M, Xia H, Seidel C, Lima e Silva R, Dong A, Hackett SF, Wang J, Howard BW, Vestweber D, Kontos CD, Peters KG, Campochiaro PA (2014) Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest 124:4564–4576. https://doi.org/10.1172/JCI74527
Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y (2003) Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci 44:393–402. https://doi.org/10.1167/iovs.02-0276
Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118:445–450. https://doi.org/10.1016/s0002-9394(14)75794-0
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. https://doi.org/10.1056/NEJM199412013312203
Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M, Eguchi S, Hori S (2005) Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology 112:806–816. https://doi.org/10.1016/j.ophtha.2004.11.045
Loukovaara S, Robciuc A, Holopainen JM, Lehti K, Pessi T, Liinamaa J, Kukkonen KT, Jauhiainen M, Koli K, Keski-Oja J, Immonen I (2013) Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol 91:531–539. https://doi.org/10.1111/j.1755-3768.2012.02473.x
Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H (2005) Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 139:476–481. https://doi.org/10.1016/j.ajo.2004.10.004
Forooghian F, Kertes PJ, Eng KT, Agron E, Chew EY (2010) Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci 51:2388–2392. https://doi.org/10.1167/iovs.09-4065
Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO (2015) The role of CTGF in diabetic retinopathy. Exp Eye Res 133:37–48. https://doi.org/10.1016/j.exer.2014.10.016
Maturi RK, Glassman AR, Josic K, Antoszyk AN, Blodi BA, Jampol LM, Marcus DM, Martin DF, Melia M, Salehi-Had H, Stockdale CR, Punjabi OS, Sun JK, Network DR (2021) Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the protocol w randomized clinical trial. JAMA Ophthalmol 139:701–712. https://doi.org/10.1001/jamaophthalmol.2021.0606
de Jong PT (2006) Age-related macular degeneration. N Engl J Med 355:1474–1485. https://doi.org/10.1056/NEJMra062326
Jager RD, Mieler WF, Miller JW (2008) Age-related macular degeneration. N Engl J Med 358:2606–2617. https://doi.org/10.1056/NEJMra0801537
Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, Souied EH, Cohen SY, Querques G, Ohno-Matsui K, Boyer D, Gaudric A, Blodi B, Baumal CR, Li X, Coscas GJ, Brucker A, Singerman L, Luthert P, Schmitz-Valckenberg S, Schmidt-Erfurth U, Grossniklaus HE, Wilson DJ, Guymer R, Yannuzzi LA, Chew EY, Csaky K, Mones JM, Pauleikhoff D, Tadayoni R, Fujimoto J (2020) Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmol 127:616–636. https://doi.org/10.1016/j.ophtha.2019.11.004
Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K (2016) Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 73:1765–1786. https://doi.org/10.1007/s00018-016-2147-8
Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M (2012) Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 122:4213–4217. https://doi.org/10.1172/JCI65157
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA (2009) An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A 106:18751–18756. https://doi.org/10.1073/pnas.0905010106
Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M (2021) Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond) 35:1305–1316. https://doi.org/10.1038/s41433-020-01377-x
Kim J, Park JR, Choi J, Park I, Hwang Y, Bae H, Kim Y, Choi W, Yang JM, Han S, Chung TY, Kim P, Kubota Y, Augustin HG, Oh WY, Koh GY (2019) Tie2 activation promotes choriocapillary regeneration for alleviating neovascular age-related macular degeneration. Sci Adv 5:eaau6732. https://doi.org/10.1126/sciadv.aau6732
Hangai M, Moon YS, Kitaya N, Chan CK, Wu DY, Peters KG, Ryan SJ, Hinton DR (2001) Systemically expressed soluble Tie2 inhibits intraocular neovascularization. Hum Gene Ther 12:1311–1321. https://doi.org/10.1089/104303401750270968
Ng DS, Yip YW, Bakthavatsalam M, Chen LJ, Ng TK, Lai TY, Pang CP, Brelen ME (2017) Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci Rep 7:45081. https://doi.org/10.1038/srep45081
Channa R, Sophie R, Bagheri S, Shah SM, Wang J, Adeyemo O, Sodhi A, Wenick A, Ying HS, Campochiaro PA (2015) Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am J Ophthalmol 159(9–19):e11-12. https://doi.org/10.1016/j.ajo.2014.09.012
Mathis T, Holz FG, Sivaprasad S, Yoon YH, Eter N, Chen LJ, Koh A, Cunha de Souza E, Staurenghi G (2023) Characterisation of macular neovascularisation subtypes in age-related macular degeneration to optimise treatment outcomes. Eye (Lond) 37:1758–1765. https://doi.org/10.1038/s41433-022-02231-y
Kienast Y, Klein C, Scheuer W, Raemsch R, Lorenzon E, Bernicke D, Herting F, Yu S, The HH, Martarello L, Gassner C, Stubenrauch KG, Munro K, Augustin HG, Thomas M (2013) Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res 19:6730–6740. https://doi.org/10.1158/1078-0432.CCR-13-0081
Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, Shen J, Dong A, Apte RS, Duh E, Hackett SF, Okoye G, Ishibashi K, Handa J, Melia M, Wiegand S, Yancopoulos G, Zack DJ, Campochiaro PA (2005) Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J 19:963–965. https://doi.org/10.1096/fj.04-2209fje
Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, Wey YS, Iwata D, Dostalek M, Moelleken J, Stubenrauch KG, Nogoceke E, Widmer G, Strassburger P, Koss MJ, Klein C, Shima DT, Hartmann G (2016) Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med 8:1265–1288. https://doi.org/10.15252/emmm.201505889
Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Chui Ming GC, Tun SBB, Wey YS, Iwata D, Dostalek M, Moelleken J, Stubenrauch KG, Nogoceke E, Widmer G, Strassburger P, Koss MJ, Klein C, Shima DT, Hartmann G (2019) Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med 11:1265–1288. https://doi.org/10.15252/emmm.201910666
Iwata D, Lundh von Leithner P, Ng YSE, Hartmann G, Shima DT (2014) Anti -VEGF/Ang2 bi-specific antibody ameliorates endotoxin-induced uveitis in mice. Invest Ophthalmol Vis Sci 55:2354–2354
Brown DM, Boyer DS, Csaky K, Vitti R, Perlee L, Chu KW, Asmus F, Leal S, Zeitz O, Cheng Y, Schmelter T, Heier JS, Investigators R (2022) Intravitreal nesvacumab (antiangiopoietin 2) plus aflibercept in diabetic macular edema: Phase 2 RUBY randomized trial. Retina 42:1111–1120. https://doi.org/10.1097/IAE.0000000000003441
Heier JS, Ho AC, Boyer DS, Csaky K, Vitti R, Perlee L, Chu KW, Asmus F, Leal S, Zeitz O, Cheng Y, Schmelter T, Brown DM (2023) Intravitreal nesvacumab (anti-angiopoietin-2) plus aflibercept in neovascular AMD: phase 2 ONYX randomized trial. J Vitreoretin Dis 7:8–15. https://doi.org/10.1177/24741264221126061
McKee S (2017) Regeneron calls time on Eylea/nesvacumab combo. Pharma Times. https://www.pharmatimes.com/news/regeneron_calls_time_on_eyleanesvacumab_combo_1212683. Accessed Aug 2023
Foxton RH, Uhles S, Gruner S, Revelant F, Ullmer C (2019) Efficacy of simultaneous VEGF-A/ANG-2 neutralization in suppressing spontaneous choroidal neovascularization. EMBO Mol Med 11:e10204. https://doi.org/10.15252/emmm.201810204
Schaefer W, Regula JT, Bahner M, Schanzer J, Croasdale R, Durr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, Schwaiger M, Stubenrauch KG, Sustmann C, Thomas M, Scheuer W, Klein C (2011) Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A 108:11187–11192. https://doi.org/10.1073/pnas.1019002108
Chakravarthy U, Bailey C, Brown D, Campochiaro P, Chittum M, Csaky K, Tufail A, Yates P, Cech P, Giraudon M, Delmar P, Szczesny P, Sahni J, Boulay A, Nagel S, Furst-Recktenwald S, Schwab D (2017) Phase I trial of anti-vascular endothelial growth factor/anti-angiopoietin 2 bispecific antibody RG7716 for neovascular age-related macular degeneration. Ophthalmol Retina 1:474–485. https://doi.org/10.1016/j.oret.2017.03.003
Sahni J, Dugel PU, Patel SS, Chittum ME, Berger B, Del Valle RM, Sadikhov S, Szczesny P, Schwab D, Nogoceke E, Weikert R, Fauser S (2020) Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the AVENUE phase 2 randomized clinical trial. JAMA Ophthalmol 138:955–963. https://doi.org/10.1001/jamaophthalmol.2020.2685
Khanani AM, Patel SS, Ferrone PJ, Osborne A, Sahni J, Grzeschik S, Basu K, Ehrlich JS, Haskova Z, Dugel PU (2020) Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol 138:964–972. https://doi.org/10.1001/jamaophthalmol.2020.2699
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V, Lai TYY, Silverman D, Regillo C, Swaminathan B, Viola F, Cheung CMG, Wong TY, Tenaya IL (2022) Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 399:729–740. https://doi.org/10.1016/S0140-6736(22)00010-1
Khanani AM, Guymer RH, Basu K, Boston H, Heier JS, Korobelnik JF, Kotecha A, Lin H, Silverman D, Swaminathan B, Willis JR, Yoon YH, Quezada-Ruiz C (2021) TENAYA and LUCERNE: rationale and design for the phase 3 clinical trials of faricimab for neovascular age-related macular degeneration. Ophthalmol Sci 1:100076. https://doi.org/10.1016/j.xops.2021.100076
Khanani AM, Kotecha A, Chang A, Chen SJ, Chen Y, Guymer R, Heier JS, Holz FG, Iida T, Ives JA, Lim JI, Lin H, Michels S, Quezada Ruiz C, Schmidt-Erfurth U, Silverman D, Singh R, Swaminathan B, Willis JR, Tadayoni R, Tenaya IL (2024) TENAYA and LUCERNE: two-year results from the phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmol. https://doi.org/10.1016/j.ophtha.2024.02.014
Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC, Hershberger VS, Pauly-Evers M, Sadikhov S, Szczesny P, Schwab D, Nogoceke E, Osborne A, Weikert R, Fauser S (2019) Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology 126:1155–1170. https://doi.org/10.1016/j.ophtha.2019.03.023
Eter N, Singh RP, Abreu F, Asik K, Basu K, Baumal C, Chang A, Csaky KG, Haskova Z, Lin H, Ruiz CQ, Ruamviboonsuk P, Silverman D, Wykoff CC, Willis JR (2022) YOSEMITE and RHINE: phase 3 randomized clinical trials of faricimab for diabetic macular edema: study design and rationale. Ophthalmol Sci 2(1):100111. https://doi.org/10.1016/j.xops.2021.100111
Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, Lin H, Loewenstein A, Mohan S, Pearce IA, Sakamoto T, Schlottmann PG, Silverman D, Sun JK, Wells JA, Willis JR, Tadayoni R, YOSEMITE and RHINE Investigators (2022) Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet 399:741–755. https://doi.org/10.1016/S0140-6736(22)00018-6
Wong TY, Haskova Z, Asik K, Baumal CR, Csaky KG, Eter N, Ives JA, Jaffe GJ, Korobelnik JF, Lin H, Murata T, Ruamviboonsuk P, Schlottmann PG, Seres AI, Silverman D, Sun X, Tang Y, Wells JA, Yoon YH, Wykoff CC, YOSEMITE and RHINE Investigators (2023) Faricimab treat-and-extend for diabetic macular edema: two-year results from the randomized phase 3 YOSEMITE and RHINE trials. Ophthalmol 131:708–723. https://doi.org/10.1016/j.ophtha.2023.12.026
ClinicalTrials.gov (2021) A study to evaluate the long-term safety and tolerability of faricimab in participants with neovascular age-related macular degeneration (AVONELLE-X). National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04777201. Accessed 21 May 2021
ISRCTN (2022) A study evaluating the effectiveness and safety of faricimab (RO6867461) in participants with polypoidal choroidal vasculopathy. ISRCTN registry. https://www.isrctn.com/ISRCTN69073386. Accessed Apr 2024
ClinicalTrials.gov (2022) A study to investigate aqueous humor and multimodal imaging biomarkers in treatment-naïve participants with diabetic macular edema treated with faricimab (ALTIMETER). National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04597918. Accessed Apr 2024
ClinicalTrials.gov (2021) A study to evaluate the long-term safety and tolerability of faricimab in participants with diabetic macular edema (RHONE-X). National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04432831. Accessed 21 May 2021
ClinicalTrials.gov (2022) A study to investigate faricimab treatment response in treatment-naive, underrepresented patients with diabetic macular edema (ELEVATUM). National Library of Medicine. https://www.clinicaltrials.gov/ct2/show/NCT05224102. Accessed Jan 2023
Eylea [package insert] (2023) regeneron pharmaceuticals, Inc., Tarrytown, NY. https://www.regeneron.com/downloads/eylea_fpi.pdf. Accessed 29 May 2024
European Medicines Agency (2021) Eylea, INN-aflibercept - summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf. Accessed 29 May 2024
Shimura M, Kitano S, Ogata N, Mitamura Y, Oh H, Ochi H, Ohsawa S, Hirakata A, Yosemite IR (2023) Efficacy, durability, and safety of faricimab with extended dosing up to every 16 weeks in Japanese patients with diabetic macular edema: 1-year results from the Japan subgroup of the phase 3 YOSEMITE trial. Jpn J Ophthalmol 67:264–279. https://doi.org/10.1007/s10384-023-00979-8
Mori R, Honda S, Gomi F, Tsujikawa A, Koizumi H, Ochi H, Ohsawa S, Okada AA, Tenaya IL (2023) Efficacy, durability, and safety of faricimab up to every 16 weeks in patients with neovascular age-related macular degeneration: 1-year results from the Japan subgroup of the phase 3 TENAYA trial. Jpn J Ophthalmol 67:301–310. https://doi.org/10.1007/s10384-023-00985-w
Singh RPH, Jeffrey S, Holz FG, Ruiz CQ, Silverman D, Ives J, Basu K, Lin H (2021) Faricimab in neovascular age-related macular degeneration: primary results from the phase 3 TENAYA and LUCERNE trials. Presented by Rishi P Singh at the Annual Meeting of the American Academy of Ophthalmology, New Orleans, LA
Guymer R, Cheung CMG, Souverain A, Yang M, Kotecha A, Margaron P (2022) Faricimab in neovascular age-related macular degeneration: year 1 results with week 12 fluid data from the phase 3 TENAYA and LUCERNE trials. Presented by Robyn Guymer at the 15th Congress of the Asia-Pacific Vitreo-retina Society, Taipei, Taiwan
Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, Rubio RG, Lai P, Group HS (2013) Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmol 120:1046–1056. https://doi.org/10.1016/j.ophtha.2012.10.014
Do DV, Sepah YJ, Boyer D, Callanan D, Gallemore R, Bennett M, Marcus DM, Halperin L, Sadiq MA, Rajagopalan N, Campochiaro PA, Nguyen QD, Group R-S (2015) Month-6 primary outcomes of the READ-3 study (Ranibizumab for Edema of the mAcula in Diabetes-Protocol 3 with high dose). Eye (Lond) 29:1538–1544. https://doi.org/10.1038/eye.2015.142
Lanzetta P (2022) Intravitreal aflibercept injection 8 mg for nAMD: 48-week results from the phase 3 PULSAR trial. Presented at: American Academy of Ophthalmology Annual Meeting; September 30–October 3, 2022; Chicago, IL
Brown D (2022) Intravitreal aflibercept injection 8 mg for DME: 48-week results from the phase 2/3 PHOTON trial. Presented at: American Academy of Ophthalmology Annual Meeting; September 30–October 3, 2022; Chicago, IL
Balaskas K, Amoaku WM, Cudrnak T, Downey LM, Groppe M, Mahmood S, Mehta H, Mohamed Q, Mushtaq B, Severn P, Vardarinos A, Yang YC (2021) Importance of anatomical efficacy for disease control in neovascular AMD: an expert opinion. Ophthalmol Ther 10:231–243. https://doi.org/10.1007/s40123-021-00342-5
ClinicalTrials.gov (2021a) A study to evaluate the efficacy and safety of faricimab in participants with macular edema secondary to central retinal or hemiretinal vein occlusion (COMINO). National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04740931. Accessed 29 May 2024
ClinicalTrials.gov (2021b) A study to evaluate the efficacy and safety of faricimab in participants with macular edema secondary to branch retinal vein occlusion (BALATON). Nationa Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04740905. Accessed 21 May 2021
Acknowledgements
Third-party medical writing assistance was provided by Karina D. Hamilton-Peel, PhD, CMPP, Adam Dagnall, DPhil, Ellen M. Ross, PhD, and Christopher A. Lamb, PhD of Envision Pharma Group, and funded by F. Hoffmann-La Roche Ltd. (Basel, Switzerland).
Funding
Financial support was provided by F. Hoffmann-La Roche Ltd. (Basel, Switzerland). The sponsor participated in the conceptualization, preparation, review, and approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Proposed the manuscript topic: HA and PDW. Drafted the manuscript: All authors. Critical revised the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Ethics approval
This review article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Hansjürgen Agostini is a consultant (institutional) for Apellis, Bayer, Novartis, Roche, and Zeiss. Francis Abreu is an employee of Genentech, Inc. Caroline R. Baumal is a consultant for Alcon and employee of Apellis. Dolly S. Chang is an employee of Genentech, Inc. Karl G. Csaky is a consultant for AbbVie, Adverum, Annexon, Cognition Therapeutics, Endogena, EyeBio, Genentech, Inc., Gyroscope, Heidelberg Engineering, Johnson & Johnson, Merck, NGM Bio, Novartis Pharma AG, Ocular Therapeutix, ReNeuron, Retrotope, and Ribomic; provides contracted research for Alexion, Annexon, Boehringer Ingelheim, Genentech, Inc., Gyroscope, Iveric Bio, and NGM Bio; and is a speaker for Genentech, Inc. Anna M. Demetriades was an employee of Genentech, Inc. during development of this manuscript. Laurent Kodjikian is a consultant for AbbVie, Alimera, Bayer, Horus, Novartis, Roche, and Thea. Jennifer I. Lim is a consultant for Aura, Cognition, Eyenuk, Luxa, Opthea, Quark, Roche/Genentech, Inc., Santen, Unity, and Viridian; has received financial support from Aldeyra, Chengdu, Janssen, NGM Bio, Regeneron, Roche/Genentech, Inc., and Stealth; and is a grant recipient from Iveric Bio and Novartis. Philippe Margaron is an employee of F. Hoffmann La Roche Ltd. Jordi M. Monés has received research funds from Apellis, Ionis Pharmaceuticals, Iveric Bio, Janssen, Kodiak Sciences, Novartis, Reneuron, and Roche; is a consultant for Annexon, Apellis, Cellcure, Iveric Bio, Lineage Cell Therapeutics, Maculogix, Novartis, PerceiveBio, Reneuron, and Roche; and has equity in EyeBio, Iveric Bio, Notal Vision, and Perceive. Tunde Peto is an advisor for Alimera, Astellas, Bayer, Heidelberg, Novartis, Optomed, OPTOS, Oxurion, and Roche. Federico Ricci is an advisor for AbbVie, Allergan, Apellis, Astellas, Bayer, Biogen, MSD, Novartis, Regeneron, and Roche. Matthias Rüth is an employee of F. Hoffmann La Roche Ltd. Rishi P. Singh is a consultant for Alcon, AsclepiX, Bausch and Lomb, Genentech, Inc., Gyroscope, Novartis, and Regeneron, and has received research sponsorship from NGM Bio. Ivaylo Stoilov is an employee of Genentech, Inc. Balakumar Swaminathan was an employee of Genentech Inc. during development of this manuscript. Jeffrey R. Willis is an employee of Genentech, Inc. Peter D. Westenskow is an employee of F. Hoffmann-La Roche Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agostini, H., Abreu, F., Baumal, C.R. et al. Faricimab for neovascular age-related macular degeneration and diabetic macular edema: from preclinical studies to phase 3 outcomes. Graefes Arch Clin Exp Ophthalmol (2024). https://doi.org/10.1007/s00417-024-06531-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00417-024-06531-9