Abstract
Purpose
The study aims to compare morphology and location of crystalline lens between acute acquired concomitant esotropia (AACE) patients and control subjects, both before and after cycloplegia.
Methods
This is a prospective and observational clinical study. Morphological and locational parameters of the crystalline lens in 53 AACE patients and 32 control subjects were assessed before and after cycloplegia using CASIA2 system, which represents the latest swept-source anterior segment optical coherence tomography. Cycloplegic refraction was recorded by administering 1% atropine in patients younger than 12 years and 1% cyclopentolate in those > 12 years old. Morphological parameters included anterior radius of curvature (ARC), posterior radius of curvature (PRC), lens thickness (LTH), and equivalent diameter of lens (LED). Locational parameters comprised lens decentration (LD) and lens tilt (LT). Comparison of these parameters before and after cycloplegia were conducted between AACE and controls. Additionally, the study analyzed and compared the changes in these parameter post-cycloplegia.
Results
Our findings suggest no significant difference in morphological parameters including ARC, PRC, LTH and LED between AACE patients and controls before or after cycloplegia. However, 2D-modeling data in the 0° meridian revealed that variation post-cycloplegia of LD (lens shift) in right eyes was different in AACE patients, measuring − 0.03(0.08) [median(interquartile range)] which was significantly distinct from the control group, exhibiting a measurement of 0.01(0.06) (z = − 2.373, p = 0.018). In left eyes, a similar trend was observed with lens shift in the 0° meridian being 0.02(0.06) in AACE, significantly differing from control group's measurement of − 0.02(0.08) (z = − 2.809, p = 0.005). Further, correlation analysis revealed that larger temporal shift of lens was associated with greater changes in ARC (r = 0.294, p = 0.006) and LTH (r = − 0.230, p = 0.031).
Conclusions
The morphological features of the crystalline lens were similar in AACE patients and controls; however, the change of lens location by cycloplegia was observed only in AACE patients, suggesting an association with excessive accommodation.
Similar content being viewed by others

Introduction
Acute acquired concomitant esotropia (AACE) is identified as a subtype of strabismus characterized by a sudden onset of concomitant esotropia with diplopia post-infancy [1], notably impacting the patients’ quality of life. Various etiologies including intracranial disease, ametropia, and abnormal anatomy of medial rectus have been proposed, yet they still remain controversial [2,3,4]. Historically, AACE was a relatively uncommon condition, accounting for only 0.3% among strabismus [5]. However, a significant increase in the incidence of AACE has been observed recently, which is considered be associated with excessive near work, particularly excessive digital device usage [6, 7]. Excessive near work leading to excessive accommodation and subsequent convergence spasm has been posited as one potential mechanism underlying AACE, gathering significant attention in recent research [8].
Although the changes of crystalline lens constitute a vital aspect of accommodation, historically, there were limited methodologies available to measure its morphological and locational parameters. Traditional techniques such as Scheimpflug photography, Purkinje images, and MRI 3D image reconstruction present challenges in widespread clinical application and in precise quantification of relevant parameters [9,10,11]. CASIA2 (Tomey GmbH), a novel second-generation anterior segment optical coherence tomography (AS-OCT), offers faster scanning speed, greater scanning depth, and improved resolution, thus facilitating the detailed quantification of both morphological and locational parameters of crystalline lens. To the best of our knowledge, studies focusing on characteristics of crystalline lens in AACE patients are scarce. The objective of our study was to investigate morphology and location of crystalline lens in AACE patients both before and after cycloplegia using CASIA2 system, and to examine associated factors.
Materials and methods
Study design
This investigation was a single-site, prospective and observational study. AACE patients were consecutively recruited at the Beijing Tongren Eye Center, Beijing Tongren Hospital between January 2021 and December 2022, and control subjects were recruited from local community between September 2021 and April 2022. This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Beijing Tongren Hospital, Capital Medical University under the approval number TRECKY2021-228. Written informed consent was secured from all participants prior to their inclusion in the study. The trial registration ID assigned to this study was ChiCTR2100053717.
Subject eligibility criteria
For the inclusion of AACE group, participants were required to meet the following criteria: (1) age of onset ≥ 5 years old, (2) acute onset of strabismus with presence of diplopia, (3) concomitant esotropia with no difference in deviation in all gaze directions, (4) corrected visual acuity of 20/20 in both eyes. For the control group, inclusion criteria comprised: (1) no strabismus assessed by alternate cover test, (2) corrected visual acuity of 20/20 in both eyes.
Participants were excluded from both the AACE and control groups if they met any of the following criteria: (1) disorder of eye movements, (2) history of intracranial or systemic diseases, (3) history of eye surgery, amblyopia, or nystagmus.
Data collection
All the patients underwent a comprehensive evaluation, which included medical history, brain and orbital computed tomography/magnetic resonance imaging, and ophthalmological and orthoptic examinations. Biometric parameters of the crystalline lens were measured with the CASIA2 swept-source optical coherence tomography (OCT) system (Tomey Corporation, Nagoya, Japan) before and after cycloplegia. Morphological parameters including anterior radius of curvature (ARC), posterior radius of curvature (PRC), lens thickness (LTH), equivalent diameter of lens (LED), locational parameters including lens decentration (LD) and lens tilt (LT) were collected in three-dimension (3D) modeling. Additionally, to account for different directions of locational parameters in 3D-modeling results, LD and LT were also obtained from 2D-modeling results of 0° meridian. The changes post-cycloplegia in both morphological and locational parameters were quantified, with the former being calculated in a 3D context and the latter in the 2D context of the 0° meridian. Cycloplegic refraction was recorded by administering 1% atropine ointment twice daily for 3 days in patients younger than 12 years and 1% cyclopentolate eye drops every 5 min for three times for those > 12 years old. Spherical equivalents (SE) were determined using the algebraic sum of the dioptric powers of the sphere and half of the cylinder. Ocular motility assessment encompassed 9 gaze directions and was conducted by experienced ophthalmologist. Deviation was measured using an alternate prism cover test performed in distance (6 m) and near (33 cm) vision.
Statistical analysis
The Shapiro–Wilk test was used to assess the normality of data. Normally distributed data were presented as mean ± standard deviation and Independent Samples T-test was used for comparative analysis. Non-normally distributed data were presented as median (interquartile range) and Mann–Whitney test was used for comparative analysis. Spearman correlation test was employed for correlation analysis of non-normally distributed data. Categorical data analysis used the Pearson Chi square test. Statistical procedures were carried out using SPSS 21.0 (SPSS Inc, Chicago, IL). P value < 0.05 (2-tailled) was considered to be statistically significant.
Results
Patients' general characteristics
The study cohort comprised 53 AACE patients (25 males and 28 females) and 32 controls (14 males and 18 females). Statistical analysis revealed no significant gender differences between the groups (p = 0.759). The mean age was 30.60 ± 12.63 years in AACE group and 31.97 ± 10.51 years in control group (p = 0.618). In the AACE group, average deviation was 26.24 ± 16.55 prism diopters (PD) in distance vision and 24.86 ± 17.20 PD in near vision.
Refractive diopter
In AACE group, three patients demonstrated mild hypermetropia, ranging from 0.75 to 1.50 diopters (D) in spherical equivalent, eighteen patients demonstrated high myopia (> 6.0D) and the others demonstrated mild myopia. While three controls were hypermetropia ranging from 0.5 to 1.75 D, six controls were high myopia and the other controls demonstrated mild myopia. The spherical equivalents (SE) of right eyes before cycloplegia were − 4.73 ± 2.54 D in AACE group, and − 3.68 ± 3.27 D in control group (p = 0.137). Similarly, the SE of left eyes before cycloplegia were − 4.47 ± 2.61 D in AACE group, and − 3.50 ± 3.34 D in control group (p = 0.179). Post-cycloplegia, no significant difference was observed between AACE and control groups in either the right eyes (− 4.06 ± 2.80 D vs − 3.43 ± 4.88 D, p = 0.376) or left eyes (− 3.92 ± 2.93 D vs − 3.31 ± 3.60 D, p = 0.429). The median change of SE post-cycloplegia in the right eyes was 0.20(0.50) D in AACE group, compared to 0.13(0.69) D in control group (p = 0.323). In the left eyes, this change was 0.13(0.41) D in AACE group and 0.13(0.50) D in control group (p = 0.884).
Morphology and location of crystalline lens
The 3D modeling results of lens morphological parameters before and after cycloplegia in AACE group and control group are presented in Table 1. Comparative analysis indicated no significant difference in the ARC, PRC, LTH, and LED between AACE and control group in both eyes before or after cycloplegia. Similarly, the changes in ARC, PRC, LTH, and LED post-cycloplegia were not significantly different.
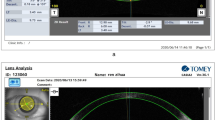
Lens locational parameters for both groups, before and after cycloplegia, derived from 3D modeling, are detailed in Table 2. Comparative analysis of LD and LT between the groups revealed no significant difference. However, variation post-cycloplegia of LD and LT was not determinable due to potential directional difference in 3D modeling. Based on data from 2D modeling results, lens locational parameters in the 0° meridian for both groups before and after cycloplegia are also presented in Table 2. While no significant difference in LD and LT were noted between the AACE and control before and after cycloplegia, a significant variation of LD post-cycloplegia (defined as “lens shift”) was observed in the right eyes, with a measurement of − 0.03(0.08) mm in AACE and 0.01(0.06) mm in control in the 0° meridian (p = 0.018), indicating a temporal shift of crystalline lens (TSL) in the AACE group. In the left eyes, the variation post-cycloplegia of LD was 0.02(0.06) mm in AACE and − 0.02(0.08) mm in control in the 0° meridian (p = 0.005), also suggesting a temporal shift of lens in the AACE group. This implies that lens location of AACE exhibited a temporal shift post-cycloplegia (Fig. 1).
A case of lens shift post-cycloplegia in AACE group. Lens decentration of the right eye (OD) was 0.07 (green line) before cycloplegia and was − 0.01 (red line) after cycloplegia. The variation of decentration (defined as lens shift) was − 0.08, which means a temporal shift of OD in the 0° meridian. Similarly, there was also a temporal shift of lens in left eyes (OS) post-cycloplegia
Correlation between TSL and ocular parameters in AACE group
For analytical purpose, positive values of lens shift in the 0° meridian in the right eyes were converted to negative values, and conversely, negative values were converted to positive values. Spearman correlation test was employed to analyze the relationship between TSL and other ocular parameters, as presented in Table 3. The study found an association between the post-cycloplegia variations in ARC (r = 0.294, p = 0.006) and LTH (r = − 0.230, p = 0.031) and temporal lens shift in AACE. As depicted in Fig. 2, patients with larger temporal shift of lens tended to show greater variations in ARC and LTH.
Discussion
AACE is characterized by a sudden onset of concomitant esotropia and is accompanied by diplopia. Recent increase of AACE has been attributed to excessive near work, particularly the intensive use of digital device such as smartphones, tablets and computers [7]. Studies have indicated a shorter working distance in AACE patients compared to age-matched controls when using smartphone or tablets [12]. This intensive near work leads to excessive accommodation and convergence. Functional vergence dysfunctions are postulated to play an important role in development of AACE and have been previously investigated [13, 14]. However, the specific impact of accommodative component remains obscure. Research has shown improvement in deviation and diplopia following the nightly application of topical cycloplegics in untreated AACE patients, even in cases persisting for over a year [8]. It was hypothesized that a 0.4% tropicamide regimen at night could mitigate the accommodative spasm, thereby reducing the associated convergence [8]. Nonetheless, existing studies have not accurately and objectively quantified the state of accommodation.
The dynamic nature of the ciliary body during accommodation, involving both contraction and relaxation, influence the crystalline lens' morphology and location. Consequently, the analysis of the crystalline lens' morphological and locational parameters offers a unique perspective in assessing the state of accommodation. In the present study, our findings did not identify any difference in lens morphology between AACE patients and control subjects. The literature on this topic is sparse, with only one case report addressing lens morphology of AACE. Frane [15] reported a case of acquired nonaccommodative esotropia in a 20-month-old child, who was a participant in a longitudinal study of ocular component development. The child's ocular parameters, including refractive error, corneal power, lens radius, lens power, and axial length were within normal ranges, aligning with 2 standard deviations of the mean of other participants. This led to the conclusion that idiopathic factors could be the underlying cause of the acute onset esotropia. Similarly, our study posits that lens morphology in AACE patients appear to be within normal ranges, though a larger sample size might be required for more definitive conclusion.
CASIA2 system, the latest swept-source AS-OCT tomography, enables measurement of tilt and decentration of crystalline lens in clinical practice. Chen [16] investigated 1097 patients awaiting cataract surgery, discovering a mean lens tilt of 5.16 degrees in the inferotemporal direction. Similarly, Li [17] observed that crystalline lens commonly tilted toward the inferotemporal direction, with an average magnitude of 4.3 ± 1.5 degrees, aligning with the findings in our study. In our current study, comparisons between AACE and control groups revealed no significant difference in lens tilt, irrespective of the measurement being taken before or after cycloplegia. Furthermore, the analysis of variations post-cycloplegia in lens tilt also indicated no significant differences between the groups.
There have been few studies focused on lens decentration until now. A mean lens decentration of 0.22 mm toward the temporal direction was observed in patients preparing for cataract surgery [16]. Li [17] reported a mean decentration of 0.17 ± 0.12 mm toward the superotemporal direction across different age groups, a finding aligning with that of our study. Investigations into the variation of lens decentration post-cycloplegia (termed 'lens shift') are scarce. As mentioned before, there is a physiological tendency for lens to decentrate toward temporal direction in most people. Consequently, we postulated that the ciliary body plays a role in maintaining this temporal decentration of the crystalline lens, a process disrupted by excessive accommodation before cycloplegia and partially restored after cycloplegia in AACE patients. Initially, our analysis indicated a trend of nasal decentration in the lens of AACE patients before cycloplegia compared to control subjects [(0.05(0.15) mm vs 0.03(0.13) mm in the right eye and − 0.04(0.21) mm vs − 0.01(0.15) mm in the left eye in 0° meridian, although not statistically significant)]. Secondly, we observed significant lens shift toward the temporal direction post-cycloplegia in both eyes of AACE patients, compared to the control group. Thirdly, a greater temporal shift of lens in AACE patients was associated with a larger variation of ARC and LTH post-cycloplegia. Given the changes of ARC and LTH constitute a major aspect of lens morphological alteration during accommodation [18], we inferred that these variations post-cycloplegia reflected the level of accommodation. This suggests that more accommodation was associated with more relief of ciliary body function following cycloplegia. Fourthly, previous studies have demonstrated the effectiveness of nightly short-acting topical cycloplegics in reducing esotropia angles in long-untreated AACE patients, corroborating the 'relief effect' of cycloplegia [8]. Finally, lens displacement creates a prism effect, where the base direction of the prism effect coincides with the direction of convex lens displacement. The temporal shift of lens post-cycloplegia in AACE patients produced a base-out prism effect, potentially ameliorating the symptoms of diplopia associated with esotropia. In essence, the post-cycloplegia relief of ciliary body function may alleviate diplopia in AACE patients. In conclusion, our study reports for the first time the phenomena of 'nasal decentration' before cycloplegia and 'temporal shift' post-cycloplegia in the lens of AACE patients, potentially linked to excessive accommodation.
There were several limitations in the present study. Firstly, not all ocular biometric parameters were investigated in the study. Given our focus on morphology and location of crystalline lens, future research could include additional parameters, such as anterior chamber depth or ocular axis length. Secondly, the limited sample size presents another constraint. Despite gaining increased attention recently, AACE remains a relatively rare form of esotropia. Our study, having enrolled 53 AACE patients, represents a substantial increase in sample size compared to previous research, yet further investigation involving a larger cohort is essential for a more comprehensive understanding of AACE characteristics. Thirdly, the absolute values of lens shift are relatively small and this might potentially impact the robustness of our findings. However, the consistent evaluation of AACE patients and controls by the same equipment and technician lends credibility to our results. Technological advances offering higher resolution may enable more definitive analyses in the future.
In conclusion, while the morphology of lens in AACE patients appears to be normal, a significant temporal shift of the lens post-cycloplegia was observed compared to controls. This shift may be linked to excessive accommodation, although the underlying mechanism warrant further investigation.
References
Clark AC, Nelson LB, Simon JW et al (1989) Acute acquired comitant esotropia. Br J Ophthalmol 73:636–638. https://doi.org/10.1136/bjo.73.8.636
Armenti ST, Miller JML, Gomez-Hassan D et al (2021) Multiple sclerosis presenting as acute acquired comitant esotropia in a pediatric patient. J aapos 25:45–47. https://doi.org/10.1016/j.jaapos.2020.08.006
Spierer A (2003) Acute concomitant esotropia of adulthood. Ophthalmology 110:1053–1056. https://doi.org/10.1016/s0161-6420(03)00102-7
Cai C, Dai H, Shen Y (2019) Clinical characteristics and surgical outcomes of acute acquired Comitant Esotropia. BMC Ophthalmol 19:173. https://doi.org/10.1186/s12886-019-1182-2
Mohney BG (2007) Common forms of childhood strabismus in an incidence cohort. Am J Ophthalmol 144:465–467. https://doi.org/10.1016/j.ajo.2007.06.011
Vagge A, Giannaccare G, Scarinci F et al (2020) Acute acquired concomitant esotropia from excessive application of near vision during the COVID-19 lockdown. J Pediatr Ophthalmol Strabismus 57:e88–e91. https://doi.org/10.3928/01913913-20200828-01
Neena R, Remya S, Anantharaman G (2022) Acute acquired comitant esotropia precipitated by excessive near work during the COVID-19-induced home confinement. Indian J Ophthalmol 70:1359–1364. https://doi.org/10.4103/ijo.IJO_2813_21
Hayashi R, Hayashi S, Machida S (2022) The effects of topical cycloplegics in acute acquired comitant esotropia induced by excessive digital device usage. BMC Ophthalmol 22:366. https://doi.org/10.1186/s12886-022-02590-w
Rosales P, Marcos S (2006) Phakometry and lens tilt and decentration using a custom-developed Purkinje imaging apparatus: validation and measurements. J Opt Soc Am A Opt Image Sci Vis 23:509–520. https://doi.org/10.1364/josaa.23.000509
Tabernero J, Benito A, Nourrit V et al (2006) Instrument for measuring the misalignments of ocular surfaces. Opt Express 14:10945–10956. https://doi.org/10.1364/oe.14.010945
Rosales P, Wendt M, Marcos S et al (2008) Changes in crystalline lens radii of curvature and lens tilt and decentration during dynamic accommodation in rhesus monkeys. J Vis 8(18):1–12. https://doi.org/10.1167/8.1.18
Van Hoolst E, Beelen L, De Clerck I et al (2022) Association between near viewing and acute acquired esotropia in children during tablet and smartphone use. Strabismus 30:59–64. https://doi.org/10.1080/09273972.2022.2046113
Zhao S, Hao J, Liu J et al (2022) Fusional vergence dysfunctions in acute acquired concomitant esotropia of adulthood with myopia. Ophthalmic Res. https://doi.org/10.1159/000527884
Campos EC (2008) Why do the eyes cross? A review and discussion of the nature and origin of essential infantile esotropia, microstrabismus, accommodative esotropia, and acute comitant esotropia. J AAPOS 12:326–331. https://doi.org/10.1016/j.jaapos.2008.03.013
Frane SL, Sholtz RI, Lin WK et al (2000) Ocular components before and after acquired, nonaccommodative esotropia. Optom Vis Sci 77:633–636. https://doi.org/10.1097/00006324-200012000-00009
Chen X, Gu X, Wang W et al (2021) Distributions of crystalline lens tilt and decentration and associated factors in age-related cataract. J Cataract Refract Surg 47:1296–1301. https://doi.org/10.1097/j.jcrs.0000000000000631
Li Z, Zhu Z, Li X et al (2021) Age-related changes in crystalline lens tilt and decentration: swept-source OCT study. J Cataract Refract Surg 47:1290–1295. https://doi.org/10.1097/j.jcrs.0000000000000632
Esteve-Taboada JJ, Domínguez-Vicent A, Monsálvez-Romín D et al (2017) Non-invasive measurements of the dynamic changes in the ciliary muscle, crystalline lens morphology, and anterior chamber during accommodation with a high-resolution OCT. Graefes Arch Clin Exp Ophthalmol 255:1385–1394. https://doi.org/10.1007/s00417-017-3663-4
Acknowledgements
We thank Dr. Wenli Yang for the technological support.
Funding
Capital Health Development Research Special Project (2022–1-2053), Young Scientists Fund of the National Natural Science Foundation of China (CN) (82101174), Beijing Hospitals Authority Youth Programme (QML20230205). The funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of Beijing Tongren Hospital, Capital Medical University under the approval number TRECKY2021-228. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Beijing Tongren Hospital, Capital Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers' bureaus, membership, employment, consultancies, stock ownership, or other equity interest, and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, W., Liu, J., Dai, W. et al. Effects of cycloplegia on crystalline lens morphology and location in acute acquired concomitant esotropia. Graefes Arch Clin Exp Ophthalmol (2024). https://doi.org/10.1007/s00417-024-06484-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00417-024-06484-z