Abstract
Purpose
This pilot study aims to comprehensively evaluate the effects of sub-Tenon’s injection of triamcinolone acetonide (STTA) on glycemic control in patients with diabetic macular edema (DME) using professional continuous glucose monitoring (CGM).
Methods
This retrospective study analyzed changes in glycemic control in 20 patients with type 2 mellitus and DME following single STTA (20 mg/0.5 mL) using The FreeStyle Libre Pro system. Professional CGM provides core CGM metrics such as the percentage of time that glucose levels fall within a target range and include the time in range (TIR) (70–180 mg/dL), time above range (TAR) (> 180 mg/dL), and time below range (TBR) (< 70 mg/dL). Outcome measures were the changes in CGM metrics (TIR, TAR and TBR) and the percentage of patients in whom TAR increased by at least 10 percentage points (ppt) 4 days before to 4 days after STTA administration.
Results
The mean CGM metrics (TIR/TAR/TBR) were 75.5%/19.9%/4.4% 4 days before STTA and 73.7%/22.4%/3.5% 4 days after STTA; the metrics 4 days before and 4 days after STTA were not significantly different (P = 0.625 for TIR, P = 0.250 for TAR, and P = 0.375 for TBR). TAR increased by more than 10 ppt in four (20%) patients treated with sulfonylurea and/or insulin.
Conclusion
Although there were no significant changes in the CGM metrics, four patients developed CGM-measured hyperglycemia after STTA for DME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction/Background
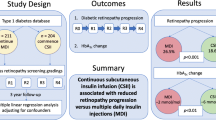
The current standard treatment for diabetic macular edema (DME) is intravitreal injection of anti-vascular endothelial growth factor (VEGF) [1]. The DRCR.net Protocol I showed that in a subgroup analysis of pseudophakic eyes, the mean change in the best-corrected visual acuity (BCVA) in eyes treated with intravitreal triamcinolone acetonide (TA) injection + prompt laser therapy was comparable to those treated with intravitreal injections of an anti-VEGF drug (ranibizumab); however, this is not level I evidence[2]. Therefore, local injection of long-acting corticosteroids, such as TA, or dexamethasone implants is frequently and widely used as an alternative therapy for eyes with DME that are refractory to anti-VEGF therapy. However, local corticosteroid injection can cause systemic and ocular side effects. Data from previous studies suggest that ophthalmologists tend to predominantly monitor only for ocular side effects, including cataract formation, ocular hypertension, and bacterial endophthalmitis [3,4,5]. In previous studies focusing on glucose levels in patients with diabetes following ocular injection of long-acting corticosteroids for DME, changes in the blood glucose (BG) level were not comprehensively assessed because the investigators used self-monitoring of BG (SMBG) or plasma sampling at specific times of the day [6,7,8,9]. Accordingly, systemic side effects of local corticosteroid injection, such as abnormal BG elevation, are likely underreported or underestimated. Continuous glucose monitoring (CGM) provides a more comprehensive picture of glycemia than either SMBG or HbA1c [10, 11]. The FreeStyle Libre Pro system (Abbott Diabetes Care, Witney, United Kingdom) consists of a factory-calibrated sensor that continuously measures glucose levels in the interstitial fluid for up to 14 days (the maximum data acquisition time for a single sensor) and a reader that retrieves stored data required for an ambulatory glucose profile (AGP) report, which is a standardized statistical and graphical information that yields several CGM metrics and identifies patterns and trends in BG levels [12]. In the current pilot study, to assess the effects of sub-Tenon’s injection of TA (STTA) on glycemic control in a comprehensive manner, we utilized the pooled CGM data obtained in clinical practice and investigated the changes in CGM metrics in patients with DME following a single STTA.
Method
Study design
This retrospective study was approved by the institutional review board of the Kobe University Graduate School of Medicine (permission number: B210299) and adhered to the tenets of the Declaration of Helsinki (7th revision). We performed a sequential review of the medical records of patients who met the inclusion criteria (glucose levels were assessed using the FreeStyle Libre Pro system 1 week before and after a single STTA for DME at Kobe University Hospital between January 2018 and November 2021). Data extracted from the medical records included the following: age, sex, eye laterality, axial length, lens status, stage of diabetic retinopathy, BCVA, intraocular pressure (IOP), central retinal thickness (CRT), macular volume (MV), history of vitrectomy, type of diabetes, HbA1c, plasma glucose (PG), C-peptide (CPR), C-peptide index (CPI), hypoglycemic drugs, systemic comorbidities, and CGM metrics. The exclusion criteria were patients with incomplete CGM data or those who had undergone prior CGM analysis.
STTA
STTA was administered on an outpatient basis. After anesthetizing the ocular surface with 4% lidocaine eye drops, disinfection of the skin around the eye and ocular surface was performed using 5% povidone–iodine and eightfold diluted PA・IODO Ophthalmic and Eye washing Solution Disinfection (Nitten Pharmaceutical Co., Nagoya, Japan). An eye lid speculum was then placed, and STTA (20 mg/0.5 mL) (Kenacort–A, Bristol-Myers Squibb, Tokyo, Japan) was administered. After injection, antibiotic eye drops and ointment were administered, the lid speculum was carefully removed, and an eyepatch was placed. The patient was instructed to remove the eyepatch the next day and continue antibiotic instillation for 4 days.
Professional CGM
A FreeStyle Libre Pro sensor was applied to the upper arm on one side one week prior to STTA administration. One week after STTA administration, the sensor, which holds data indefinitely, was removed, and data were downloaded and analyzed using the FreeStyle LibreView software. Consequently, we obtained data on core CGM metrics and AGP in each patient. The CGM metrics used in this study was the percentage of time that sensor glucose levels fell within a target range, including the time in range (TIR) (70–180 mg/dl), time above range (TAR) (> 180 mg/dL), and time below range (TBR) (< 70 mg/dL) as is evident from the consensus among diabetes specialists [12]. The FreeStyle Libre Pro system is a retrospective CGM system that allows patients and physicians to review the data only after wearing the sensor.
Outcome measures
Primary outcome measures were the changes in CGM metrics (TIR, TAR, and TBR) and the percentage of patients in whom the TAR increased by at least 10 percentage points (ppt) 4 days before to 4 days after STTA. In order to account for the potential stress associated with sensor placement or STTA procedure, we have excluded data from at least the first two days following sensor attachment and from the day of STTA. Secondary outcome measures were the changes in the logarithm of the minimum angle of resolution (logMAR) BCVA, IOP, CRT, and MV from before STTA to 1 month after STTA. BCVA was measured using a standard Landolt chart, and the decimal BCVA values were converted to logMAR BCVA values for statistical analyses. CRT and MV values were automatically calculated by the built-in software using the Macular Cube 200 × 200 scan data acquired by optical coherence tomography (Cirrus HD-OCT 5000, Carl Zeiss Meditec, Tokyo, Japan).
Statistical analyses
Values were described as medians with interquartile ranges (IQRs) unless otherwise indicated. The Wilcoxon test was used to compare the time of each variable. To compare the differences in variables between two groups (i.e., patients with and without increased TAR after STTA), the Mann–Whitney U test or Chi–square test was used as appropriate. Statistical analyses were performed using MedCalc v.20.027 software (MedCalc Software, Ostend, Belgium). P-values < 0.05 were considered significant.
Results
Of the 23 patients who met the inclusion criteria, 3 were excluded due to incomplete CGM data. Accordingly, data from the remaining 20 patients were used for the analyses. The characteristics of the 20 patients are summarized in Table 1. The prescribed hypoglycemic drugs of the patients included dipeptidyl peptidase-4 inhibitors (85%), biguanides (65%), sulfonylurea (35%), insulin (15%), thiazolidinediones (10%), alpha-glucosidase inhibitors (10%), sodium-glucose cotransporter 2 inhibitors (10%), meglitinides (5%), and glucagon-like peptide-1 receptor agonist (5%). Systemic comorbidities included hypertension (45%), dyslipidemia (20%), chronic kidney disease (10%), angina pectoris (10%), and others (50%).
Changes in CGM metrics over time are shown in Fig. 1. The median (IQR) TIR (%) was 72% (56.75%, 82%) on day − 4, 53% (48.75%, 86%) on day − 3, 76.5% (59.5%, 82%) on day − 2, 75.5% (70%, 92%) on day − 1, 79% (65%, 87.5%) on day 0, 78% (45%, 93%) on day + 1, 76% (52%, 93%) on day + 2, 68% (50%, 89.75%) on day + 3, and 69% (52%, 83.25%) on day + 4 (the date of STTA administration was counted as day 0). The median (IQR) TAR (%) was 23.5% (3.25%, 43.25%) on day − 4, 38% (11%, 50.25%) on day − 3, 20.5% (10.25%, 33.5%) on day − 2, 8% (2%, 29.25%) on day − 1, 19.5% (9.25%, 35%) on day 0, 20.5% (5.75%, 55%) on day + 1, 22% (7%, 48%) on day + 2, 31.5% (8.75%, 41.5%) on day + 3, and 24% (10.5%, 36.5%) on day + 4. The median (IQR) TBR (%) was 0% (0%, 0.5%) on day − 4, 1% (0%, 4%) on day − 3, 0% (0%, 7.25%) on day − 2, 0% (0%, 4.75%) on day − 1, 0% (0%, 0%) on day 0%, 0% (0%, 3%) on day + 1, 0% (0%, 0.25%) on day + 2, 0% (0%, 0.75%) on day + 3, and 0% (0%, 0%) on day + 4. There was not a significant difference between days − 4 and − 1 and between days + 1 and + 4 in each CGM metric (P = 0.625 for TIR, P = 0.250 for TAR, and P = 0.375 for TBR) (see Supplementary Materials).
Changes in CGM metrics following STTA for DME. A A line graph shows changes in the median CGM metrics over time following STTA administration for DME. B A box plot comparing CGM metrics between days − 4 to − 1 and days + 1 to + 4. A significant change was not observed in each CGM metric (P = 0.625 for TIR, P = 0.250 for TAR and P = 0.375 for TBR). CGM, continuous glucose monitoring; STTA, sub-Tenon’s injection of triamcinolone acetonide; DME, diabetic macular edema; TIR, time in range; TAR, time above range; TBR, time below range
The TAR in four (20%) patients increased by more than 10 ppt from four days before to four days after STTA; all were maintained on sulfonylureas and/or insulin. The characteristics of these patients are shown in Table 2. In the comparison of variables between the increased TAR group (n = 4) and non-increased TAR groups (n = 16), a significant difference was observed in age, sex, and insulin use. The median (IQR) age was 77 (76.75, 77.75) and 71 (63, 73.25) years in the increased and non-increased TAR groups, respectively (P = 0.014). Women comprised 75% and 50% of the patients in the increased and non-increased TAR groups, respectively (P = 0.038). The proportion of patients who used insulin in the increased and non-increased TAR groups was 50% and 6%, (P = 0.033). The median (IQR) of other variables in the increased and non-increased TAR groups was 7.85% and 7.00% for HbA1c (P = 0.105), 169 mg/dL and 126 mg/dL for PG (P = 0.219), 1.85 ng/mL and 2.37 ng/mL for CPR (P = 0.643), and 0.896 and 1.877 for CPI (P = 0.254), respectively. The proportion of patients who used sulfonylureas in the increased and non-increased TAR groups was 50% and 31%, respectively (P = 0.493). The daily glucose profiles of representative cases with or without increased TAR following STTA are presented in Figs. 2 and 3. Hyperglycemia occurred in the afternoon and evening in three (75%) patients with increased TAR.
Daily glucose profiles of a representative case without increased TAR following STTA. Blood glucose levels remained within normal range even after STTA administration. A single green arrow indicates the time point when STTA was performed. TAR, time above range; STTA, sub-Tenon’s injection of triamcinolone acetonide
Daily glucose profiles of a representative case with increased TAR following STTA. Blood glucose levels were elevated above normal range predominantly in the afternoon and evening after STTA. A single green arrow indicates the time point when STTA was performed. TAR, time above range; STTA, sub-Tenon’s injection of triamcinolone acetonide
One month after STTA administration, the median (IQR) logMAR BCVA, IOP (mmHg), CRT (µm) and MV (mm3) was 0.261 (0.128, 0.429), 16 (15.1, 16.8), 385 (307.5, 486.25), and 12.2 (11.275, 13.5), respectively. There was a significant difference in the CRT before and 1 month after STTA (P < 0.001), but not in the logMAR BCVA (P = 0.326), IOP (P = 0.190), and MV (P = 0.105).
Discussion
In the current study, CGM metrics (TIR, TAR, and TBR) did not significantly change after STTA, indicating that the overall effect of STTA on glycemic control is modest. The unchanged glycemic control after STTA in this study is consistent with those previously reported [6]. Feldman-Billard et al. analyzed BG changes in 67 patients with DME after a single 4-mg intravitreal TA injection by monitoring 6–10 daily capillary BG measurements and concluded that a 4-mg intravitreal TA injection would not generally change glycemic levels in patients with diabetes [6]. Kadeli et al. prospectively evaluated the influence of STTA (40 mg) on PG (fasting PG [FPG] and HbA1c), cortisol, and adrenocorticotrophic hormone (ACTH) in 33 patients with type 2 diabetic mellitus and DME; they found that STTA did not significantly change the FPG, HbA1c, cortisol, and ACTH levels as compared with those in the controls (ST injection of saline solution) [8]. Additionally, Posch-Pertl et al. assessed the PG profiles of nine patients with type 2 diabetes mellitus and DME who had been implanted with intravitreal dexamethasone (0.7 mg) and reported that glucose levels using a 7-point PG profile did not show a significant change after intravitreal dexamethasone implantation at any single time point (1–3 days, 1 week and 1 month) [9]. However, in a clinical trial evaluating glucose levels following intra-articular injection of TA crystalline suspension in patients with type 2 diabetes mellitus with knee osteoarthritis using CGM, the mean daily CGM-measured glucose level increased from baseline (days − 3 to − 1) to days 1–3 by 37.1 mg/dL (from 161.7 mg/dL to 198.8 mg/dL), suggesting that CGM could assess changes in glucose levels induced by local TA injection that conventional SMBG may fail to reveal [13].
Notably, 20% of the patients had a greater than 10 ppt increase in TAR following STTA in the current study. As TAR is a metric that includes time, it could more accurately reflect BG changes than repeated SMBG. As mentioned above, patients with type 2 diabetes and knee osteoarthritis exhibited increased daily CGM-measured glucose levels following intra-articular TA injection [13]. Additionally, the daily glucose profiles of patients with increased TAR in our study showed hyperglycemia in the afternoon and evening. This pattern of hyperglycemia is consistent with that reported by Burt et al., who investigated the circadian effects of prednisolone on glucose concentrations in hospitalized patients with chronic obstructive pulmonary disease without known diabetes using CGM [14]. The elevation of glucose levels after STTA was previously reported. Toda et al. analyzed FPG levels before vs. after STTA in 16 patients with type 2 diabetes and DME and found that 3 (19%) patients had FPG levels equal to or greater than 200 mg/dL after administration [7]. Given the clinical impact of hyperglycemia, it should be noted that the overall effect of STTA on glycemic control is not significant; however, some patients may develop hyperglycemia after STTA. The daily glucose profiles of the representative case with increased TAR following STTA (Fig. 3) clearly shows that SMBG values at specific time points would over- or under-estimate the impact of STTA on the BG level. If a blood sample was taken at a time when the glucose level is close to its peak, an internist who was not informed of previous STTA from an ophthalmologist might decide to shift to a more aggressive diabetes management strategy, which may lead to severe hypoglycemia as the effects of STTA subside with time. Although we searched for predictors of cases with increased TAR following STTA, the number of cases was insufficient to perform a logistic analysis. However, the insulin secretion capacity might be relevant to this event because all four patients with increased TAR were treated with sulfonylureas and/or insulin, and three (75%) had a CPI < 1.1. Meanwhile, one patient was undergoing hormone therapy for a neuroendocrine tumor, and the excess steroid hormones may have led to the increased TAR.
We had no alternative but to conduct a small retrospective study this time because no preceding studies have not been conducted so far. Therefore, most of the limitations in this study stem from its retrospective nature and the small sample size. First, the sample size was probably too small to assess the statistical significance of the rare side effects (i.e., CGM-measured hyperglycemia after STTA) although we do not thick that this detracts from the clinical relevance of this study. Second, it is unknown whether intravitreal TA injection or an intravitreal dexamethasone implant for DME shows similar daily CGM-measured glucose levels as those with STTA. Third, we did not determine the reproducibility of the daily glucose profiles following STTA. Fourth, the threshold level in TAR increase does not have a basis in the evidence and should, therefore, be subject to validation in future studies. Finally, the maximum duration of sensor use of the current Libre Pro device is 14 days; therefore, the long-term effects of STTA on daily glucose profiles remains unknown. Further prospective analyses and advances in CGM technology could resolve these problems and contribute to highly accurate predictions of blood glucose fluctuations.
In conclusion, we analyzed CGM data from 20 patients with DME following a single STTA and obtained novel insights into the changes in glucose levels that conventional SMBG failed to reveal. STTA for DME does not significantly change the CGM metrics. However, it should be noted some patients may develop CGM-measured hyperglycemia after STTA.
References
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, Maia M, Mathenge W, Moreker S, Muqit MMK, Resnikoff S, Verdaguer J, Zhao P, Ferris F, Aiello LP, Taylor HR (2018) Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 125:1608–1622. https://doi.org/10.1016/j.ophtha.2018.04.007
Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117(1064–1077):e1035. https://doi.org/10.1016/j.ophtha.2010.02.031
Chew EY, Glassman AR, Beck RW, Bressler NM, Fish GE, Ferris FL, Kinyoun JL, Diabetic Retinopathy Clinical Research N (2011) Ocular side effects associated with peribulbar injections of triamcinolone acetonide for diabetic macular edema. Retina 31:284–289. https://doi.org/10.1097/IAE.0b013e3181f049a8
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM, Ozurdex MSG (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121:1904–1914. https://doi.org/10.1016/j.ophtha.2014.04.024
Maeda Y, Ishikawa H, Nishikawa H, Shimizu M, Kinoshita T, Ogihara R, Kitano S, Yamanaka C, Mitamura Y, Sugimoto M, Kondo M, Takamura Y, Ogata N, Ikeda T, Gomi F (2019) Intraocular pressure elevation after subtenon triamcinolone acetonide injection; Multicentre retrospective cohort study in Japan. PLoS ONE 14:e0226118. https://doi.org/10.1371/journal.pone.0226118
Feldman-Billard S, Chibani A, Heron E (2008) Intravitreal triamcinolone and blood glucose. Ophthalmology 115(917–917):e912. https://doi.org/10.1016/j.ophtha.2007.11.012
Toda J, Fukushima H, Kato S (2009) Systemic complications of posterior subtenon injection of triamcinolone acetonide in type 2 diabetes patients. Diabetes Res Clin Pract 84:e38-40. https://doi.org/10.1016/j.diabres.2008.10.020
Kaderli B, Kivanc SA, Inan UU, Ersoy C, Yucel AA, Yilmaz S, Avci R (2014) Effect of posterior subtenon injection of 40 mg of triamcinolone acetonide on glycemic control and serum cortisol and adrenocorticotropic hormone in diabetic patients. Eur Rev Med Pharmacol Sci 18:2609–2614
Posch-Pertl L, Schwetz V, Michelitsch M, Rabensteiner J, Holler V, Gasser-Steiner V, Weger M, Sourij H (2018) Effect of dexamethasone intravitreal implant on blood glucose, hypothalamic-pituitary-adrenal axis function and vascular endothelial growth factor serum levels in patients with diabetic macular oedema. Acta Ophthalmol 96:e543–e544. https://doi.org/10.1111/aos.13615
Cappon G, Vettoretti M, Sparacino G, Facchinetti A (2019) Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J 43:383–397. https://doi.org/10.4093/dmj.2019.0121
Krakauer M, Botero JF, Lavalle-Gonzalez FJ, Proietti A, Barbieri DE (2021) A review of flash glucose monitoring in type 2 diabetes. Diabetol Metab Syndr 13:42. https://doi.org/10.1186/s13098-021-00654-3
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ 3rd, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Norgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer SA, Phillip M (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42:1593–1603. https://doi.org/10.2337/dci19-0028
Russell SJ, Sala R, Conaghan PG, Habib G, Vo Q, Manning R, Kivitz A, Davis Y, Lufkin J, Johnson JR, Kelley S, Bodick N (2018) Triamcinolone acetonide extended-release in patients with osteoarthritis and type 2 diabetes: a randomized, phase 2 study. Rheumatology (Oxford) 57:2235–2241. https://doi.org/10.1093/rheumatology/key265
Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN (2011) Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 96:1789–1796. https://doi.org/10.1210/jc.2010-2729
Funding
Open access funding provided by Kobe University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial interests
Dr. Yushi Hirota and Wataru Ogawa received payments from Abbott Japan within the past 36 months for matters unrelated to this study.
Non-financial interests
Dr. Sentaro Kusuhara is an editor of Graefe’s journal.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board (IRB) of the Kobe University Graduate School of Medicine (permission number: B210299).
Consent to participate
Since the analysis was retrospective, a waiver of informed consent was granted.
Conflict of interest
Sentaro Kusuhara—although the corresponding author is the editor of the journal, there was no involvement with the peer review process for this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sotani-Ogawa, R., Kusuhara, S., Hirota, Y. et al. Continuous glucose monitoring metrics following sub-Tenon’s injection of triamcinolone acetonide for diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 262, 449–456 (2024). https://doi.org/10.1007/s00417-023-06275-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06275-y