Abstract
Purpose
This is, to our knowledge, the first network meta-analysis aiming to compare all treatment modalities for myopic choroidal neovascularization (CNV).
Methods
After the electronic databases were searched, two independent reviewers screened titles, abstracts, full-texts, and extracted information. Primary endpoints were change in visual outcome and central retinal thickness. We used a network meta-analysis to compare treatment outcomes in the early (≤ 6 months) and late (> 6 months) phase.
Results
We included 34 studies (2,098 eyes) in our network meta-analysis. In the early phase, the use of anti-VEGF led to a gain of 14.1 letters (95% CI, 10.8–17.4) compared to untreated patients (p < 0.0001), 12.1 letters (95% CI, 8.3–15.8) to photodynamic therapy (PDT) (p < 0.0001), 7.5 (95% CI, 1.2–13.8) letters to intravitreal triamcinolone acetonide (TCA) (p = 0.019), and − 2.9 letters (95% CI, − 6.0–0.2) to the combination of anti-VEGF and PDT (p = 0.065). In the later phase, these results were largely maintained. There were no significant differences in visual outcomes between patients treated with 1 + PRN and 3 + PRN. However, the 1 + PRN group received 1.8 (SD 1.3), while the 3 + PRN group received 3.2 (SD 0.9) injections within 12 months (p < 0.0001).
Conclusion
This network meta-analysis confirms that anti-VEGF is the most effective treatment for myopic CNV using the 1 + PRN treatment strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Pathologic myopia is a major cause of blindness affecting almost 2% of the population worldwide. Although the definition of pathologic myopia has not been standardized yet, it is usually classified as a refractive error of less than − 6.00 diopters and an axial length of ≥ 26.5 mm combined with degenerations of the sclera, choroid, and retina [1]. One of the most common complications of pathologic myopia leading to blindness is the development of choroidal neovascularization (CNV). Myopic CNV is associated with a poor prognosis if untreated leading to a decline in visual acuity. More than a third of the patients affected by myopic CNV are at risk of developing myopic CNV in the unaffected eye within 8 years [2].
For a long time, verteporfin photodynamic therapy (PDT) was the only treatment approved for myopic CNV [3]. PDT treatment was able to stabilize visual acuity; however, long-term results were discouraging. The development of VEGF inhibitors revolutionized treatment of myopic CNV and soon superseded PDT as the new gold standard treatment [4].
Several studies [5,6,7] have been performed to compare different treatments for myopic CNV; however, no common comparator was used. Therefore, this study is aimed at comparing the efficacy of different treatment options for myopic CNV using a network meta-analysis.
Methods
Literature search
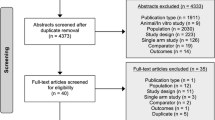
The literature search was performed by an experienced medical information specialist (BW). The following electronic databases were searched for publications from database inception to July 2020: MEDLINE, Embase, Cochrane Central Register of Controlled Trials and Web of Science (SCI-Expanded, SSCI, CPCP-S and ESCI) using free term and controlled term formulations. Databases were searched for the following keywords: “myopic choroidal neovascularization” AND “treatment”;—AND “aflibercept”;—AND “bevacizumab”;—AND “ranibizumab”;—AND “conbercept”;—AND “PDT”;—AND “photodynamic therapy”;—AND “triamcinolone”;—AND “surgery”;—AND “sham”. We limited our search to articles published in English. The bibliographies of identified articles were scanned to identify additional manuscripts that were missed in our previous database search. The protocol of this network meta-analysis was not registered in PROSPERO. This review followed the Cochrane handbook [8] and the PRISMA for network meta-analysis checklist (see Fig. 1 and Supplementary Table 1) [9].
PRISMA flow diagram adapted by Page et al. [9]
Study eligibility criteria
All study types (i.e., randomized controlled, prospective and retrospective cohort studies, cross-sectional, case–control, and survey and surveillance reports) comparing treatments for myopic choroidal neovascularization were included. Studies had to report ≥ 2 treatment groups, original data on adult patients (≥ 18 years), and a sample size ≥ 10 and had to be published in English.
Abstracts and conference proceedings not published in peer-reviewed journals were not included.
Study selection
Two reviewers (LP and LG) independently screened references for inclusion. Included references underwent dual abstract and subsequent full-text review to decide on final inclusion or exclusion of the study. Disagreements were resolved by discussion. The online software “Rayyan” [10, 11] was used for abstracts and full-text screening.
Data extraction
Two investigators (LP and LG) independently extracted the title, name of authors, year of publication, study design, sample size, treatment, best-corrected visual acuity (BCVA) at baseline and follow-up, central retinal thickness (CRT) at baseline and follow-up, number of treatments, and demographic data. BCVA has been converted to ETDRS letters to enable comparison between the different ways of reporting. Further, descriptive data such as country of origin, definition of myopic CNV, minimal axial length, inclusion and exclusion criteria, and pretreatment were documented. These data were recorded in a Microsoft Excel (Microsoft Cooperation) spreadsheet.
Data analysis
For the analysis, the change in visual outcomes was used, which is given by the mean difference between baseline and follow-up for each treatment group. Most included studies provided means and standard deviations at baseline and for specific follow-up dates. Then, the mean difference can be easily calculated, and for the standard deviation of change, the Cochrane Handbook for Systematic Reviews of Interventions [12] was followed, assuming a correlation of 0.6. This value was chosen due to a Methods Research Report [13] that refers to a median of 0.59 for correlation of change from baseline. Furthermore, one of the included studies [14] reported a correlation of 0.646. As a sensitivity analysis, no correlation was assumed. If not directly specified, further measures were taken into account to calculate the change and the corresponding standard deviation in visual outcomes. This included the use of p values, confidence intervals, and as a final option, if the standard deviation for the baseline was given but the standard deviation for the follow-up date was missing, the baseline value was used as a surrogate. The network meta-analysis was based on a random effects model, and correlation in multi-arm studies was considered [15]. The common heterogeneity variance \({\tau }^{2}\) used in the random effects model was estimated by a generalized DerSimonian-Laird estimator [16]. To assess inconsistency, the between-designs Q statistic was calculated based on a full design-by-treatment interaction random effects model [17]. The fitted models were used to compare the efficacy of different treatments, for two distinct time points and two separate outcomes.
The follow-up dates were grouped into two phases, the first describing treatments in the earlier phase, one to six months. If more than one follow-up date was specified, priority was given to 3 months, then 6 months, and 1 month as the last option. The second time point was considered the later phase, where 24 months, then 12 months, and as the final option, any follow-up dates beyond 24 months were prioritized.
The outcomes determining the efficacy of the treatment referred to the visual improvement measured by the BCVA in letters on the one hand and to the anatomical recovery measured by the CRT in micrometers on the other hand.
Furthermore, subgroup analysis for the different anti VEGFs was performed for the same time points and outcomes. In the primary analysis, we did not distinguish between one initial injection followed by a pro re nata approach (1 + PRN) and three initial injections followed by a PRN approach (3 + PRN); furthermore, we performed a separate pairwise meta-analysis to evaluate possible differences between the two treatment regimens. To compare the number of treatments, we used a two-sample t-test with Welch–Satterthwaite correction on pooled standard deviations and means.
A p value of less than 0.05 was considered statistically significant. All analyses were performed in R, Version 4.1.3 [18].
Results
Our literature search yielded 1,156 articles (see Fig. 1). 166 full text articles of these were screened for eligibility. We included 64 studies for our qualitative and 34 studies for our quantitative analysis (see Tables 1 and 2).
Study characteristics
In the quantitative analysis, we included 34 studies comprising 2,098 eyes from 2,059 patients. 29 studies had two arms and 5 three arms. In the qualitative analysis comprising 64 studies and 4,641 eyes, 52 were two-arm studies, 9 three-arm studies, one was a four-arm study, and 2 were five-arm studies.
Outcome in the earlier phase (≤ 6 months)
The evidence network for BCVA in the early phase included 10 studies, representing 5 treatments and no treatment (see Fig. 2).
The structure of the network comparing different treatments regarding BCVA in the early phase (< 6 month). The numbers represent the numbers of direct comparisons, while the thickness of the lines is proportional to the inverse standard error of the estimates. BCVA, best-corrected visual acuity; PDT, photodynamic treatment; TCA, intravitreal triamcinolone acetonide; VEGF, vascular endothelial growth factors
In the early phase (≤ 6 months), patients treated by anti-VEGF gained on average 14.1 letters (95% CI, 10.8–17.4) more compared to untreated patients (p < 0.0001). Likewise, patients treated by anti-VEGF gained on average 12.1 letters (95% CI, 8.3–15.8) more than patients treated by PDT (p < 0.0001) and 7.5 letters (95% CI, 1.2–13.8) more than patients treated by intravitreal triamcinolone acetonide (TCA) (p = 0.019). The combination of PDT and anti-VEGF did not result in better visual outcome (MD − 2.9; 95% CI, − 6.0–0.2; p = 0.065) (see Fig. 3).
The other treatment modalities showed less favorable results in the early phase (≤ 6 months). Patients treated with TCA had gained in the mean 6.6 letters (95% CI, − 0.5–13.7) more compared to untreated patients (p = 0.068). The PDT treatment group had no significant change in visual acuity compared to the untreated group (MD − 2.01 letters; 95% CI, − 7.0 − 3.0; p = 0.430). There was no evidence of inconsistency within the network (p = 0.204).
For central retinal thickness (CRT) in the early phase, only 2 studies were included (one two arm and one three arm study). The resulting network structure is therefore very simple (see Fig. 4). Even though the number of comparisons is small, the fitted network meta-analysis shows similar results compared to the analysis of BCVA. We can observe a significant decrease in CRT in patients treated with anti-VEGF compared to untreated patients (66.8 μm; 95% CI, 40.2 − 93.4; p < 0.0001) and patients treated with PDT (27.7 μm; 95% CI, 16.1–39.3; p < 0.0001). The combination treatment of PDT and anti VEGF therapy had a significant larger decrease in CRT than patients treated solely with anti-VEGF (12.0 μm; 95% CI, 21.4–2.6; p = 0.013) (see Fig. 5). Due to the small number of included studies, it is not reasonable to assess inconsistency.
The structure of the network comparing different treatments regarding BCVA in the early phase (< 6 month). The numbers represent the numbers of direct comparisons, while the thickness of the lines is proportional to the inverse standard error of the estimates. PDT, photodynamic treatment; TCA, intravitreal triamcinolone acetonide; VEGF, vascular endothelial growth factors
Patients treated with 1 + PRN anti-VEGF gained 0.8 letters less (95% CI, − 2.8–4.5; p = 0.652) and their CRT decreased 20.0 μm less (95% CI, − 44.7–4.6; p = 0.111) compared to patients treated with 3 + PRN.
Outcome in the later phase (> 6 months)
Concerning the long-term results of BCVA, the evidence network consists of 16 studies, comparing five different treatments as well as no treatment (see Fig. 6). In the anti-VEGF treatment group, the early outcome could be maintained in the long-term analysis with a mean estimated gain of 28.4 letters (95% CI, 22.7–34.1) when compared to untreated patients (p < 0.0001). Patients treated with anti-VEGF gained 13.1 letters (95% CI, 9.7–16.5) more than patients treated with PDT (p < 0.0001) and 7.5 letters (95% CI, − 1.0–16.0) more than patients treated with TCA, although this was not significant (p = 0.084). There was no significant difference between the anti-VEGF group and the combination (PDT and anti VEGF) group (− 0.02; 95% CI, − 3.9–3.8; p = 0.991). Also, the gain of 9.91 letters (95% CI, − 11.27–31.08) in the surgical group compared to anti-VEGF treatment stayed not significant (see Fig. 7). We did not observe inconsistency in the network (p = 0.328).
The structure of the network comparing different treatments regarding BCVA in the early phase (< 6 month). The numbers represent the numbers of direct comparisons, while the thickness of the lines is proportional to the inverse standard error of the estimates. BCVA, best-corrected visual acuity; PDT, photodynamic treatment; TCA, intravitreal triamcinolone acetonide; VEGF, vascular endothelial growth factors
Central retinal thickness in the later phase was compared using 5 studies with three treatments. Therefore, the network structure shows a triangle shape (see Fig. 8). The network meta-analysis showed no significant difference in the anti-VEGF group compared to the PDT group (10.4 μm; 95% CI, − 37.1–57.8) and no difference to the combination (PDT and anti-VEGF) group (25.3 μm; 95% CI, − 56.7–107.2) (see Fig. 9). Again, this network did not show signs of inconsistency (p = 0.447).
The structure of the network comparing different treatments regarding BCVA in the early phase (< 6 month). The numbers represent the numbers of direct comparisons, while the thickness of the lines is proportional to the inverse standard error of the estimates. PDT, photodynamic treatment; VEGF, vascular endothelial growth factors
Forrest plot comparing change in central retinal thickness after six months in the anti-VEGF treatment group compared to the other treatment groups. CI, confidence interval; MD, mean difference; PDT, photodynamic treatment; TCA, intravitreal triamcinolone acetonide; VEGF, vascular endothelial growth factors
Patients treated with 1 + PRN anti-VEGF gained 0.7 letters (95% CI, − 2.3–3.8, p = 0.635) compared to the patients treated by 3 + PRN, and their CRT decreased by 3.2 (95% CI, − 15.1–21.4, p = 0.734).
Differences in anti-VEGF drugs
We compared the change in BCVA of different anti-VEGF drugs in the early phase including 8 studies and in the later phase including 13 studies. There was no significant difference in letters gained in patients receiving bevacizumab compared to aflibercept (p = 0.222), ranibizumab (p = 0.124), and conbercept (p = 0.572) in the early phase, the same was seen in the later phase (p = 0.250, p = 0.265, respectively, p = 0.382).
For CRT, we investigated 5 studies for both time points. In the early phase, CRT decreased significantly in patients receiving aflibercept compared to bevacizumab (12.1 μm; 95% CI, 3.0–21.2; p = 0.009). There was no significant difference in the change of CRT between bevacizumab, ranibizumab (7.6 μm; 95% CI, − 13.3–28.5), and conbercept (− 5.4 μm; 95% CI, − 41.5–30.8). Moreover, there was also no significant difference observed comparing long-term results of the different anti-VEGF factors.
Treatment strategies
4 studies compared 1 + PRN and 3 + PRN treatment strategies. Patients treated with 1 + PRN received 1.8 (SD 1.3) injections within 12 months, while patients with 3 + PRN received 3.2 (SD 0.9) injections (p < 0.0001).
Also, the number of injections in patients receiving PDT + anti-VEGF versus solely anti-VEGF was compared. Patients receiving combination treatment required 2.2 (SD 1.5) injections, and patients receiving only anti-VEGF treatment required 2.6 (SD 1.3). This difference was not significant (p = 0.155).
Other treatments
Other treatment options for myopic CNV had too few comparators for our quantitative analysis. A summary statement for each option is given in our supplementary table.
Discussion
This network meta-analysis showed that the intravitreal injection of anti-VEGF using the regimen of 1 + PRN is an effective treatment for myopic CNV, with both short- and long-term beneficial results.
Intravitreal injection of anti-VEGF is considered the gold standard treatment for myopic CNV, which is confirmed in this network meta-analysis. In diabetic macular edema, aflibercept is proposed to lead to a greater improvement in visual acuity compared to other VEGF inhibitors in patients with low baseline BCVA (< 69 letters) [24]. Therefore, we compared the different VEGF inhibitors, i.e., bevacizumab, ranibizumab, aflibercept, and conbercept. However, we found no difference between the VEGF inhibitors. Aflibercept led to a larger decrease in CRT, but this had no impact on visual acuity. Due to small sample sizes, we did not differentiate between low and high baseline BCVA. Future research should investigate this.
We then compared the different treatment strategies for VEGF inhibitors. There was no significant difference in letters gained whether three injections were administered consecutively as loading dose or only one. However, patients treated with 1 + PRN required significantly less injections than patients with 3 + PRN. This outcome might indicate that the 3 + PRN treatment strategy leads to an overtreatment. Future research should investigate in subgroup analysis, whether this is true for different VEGF inhibitors.
Combining anti-VEGF treatment with PDT showed a slightly greater decrease in CRT in the early phase, although the absolute difference of 12 μm may be clinically insignificant. There was a tendency to gain more estimated letters, but this was not significant. In the long-term results (> 6 months), change in BCVA and CRT was the same for anti-VEGF treatment and the combination of PDT and anti-VEGF. There was no difference between these two groups in the number of injections required within 12 months. Considering the absence of randomized controlled trials and the lack of differing results, anti-VEGF monotherapy seems the more reasonable first line treatment.
Intravitreal TCA was inferior to anti-VEGF in terms of letters gained in the short-term analysis, but no statistical difference was seen in long-term analysis. Intravitreal TCA is known to cause an IOP increase in nearly one-third of all patients and has a high prevalence of cataract formation and progression over time. In regard of these known side effects, anti-VEGF appears to be the more favorable choice.
When comparing the previous gold standard PDT for myopic CNV to anti-VEGF, patients with PDT gained significantly less letters over all time periods. This strengthens the use of anti-VEGF over PDT.
In our systematic review, it seems unlikely that other treatment options for myopic CNV show similar visual improvement compared to intravitreal VEGF inhibitors, although patient numbers were too small to prove this in our quantitative network meta-analysis (see supplementary table 2).
The numbers of complications were too small to calculate the risk of complications. In Table 2, we reported complications rates, which were low in general. Surgical interventions had the highest complication rates. Intravitreal steroids, as known, showed an increase of intraocular pressure and cataract progression. In patients with intravitreal VEGF inhibitors, some patients showed corneal erosions and dry eye symptoms after injection. Not all studies reported on these relatively common adverse events, which is the reason why no numbers can be given. The same applies to IOP elevation, as most studies did not measure IOP after injection. There were three (0.001%) reports of retinal detachment after intravitreal injection and one (0.0004%) case of sterile vitritis in the studies reporting on complications.
This network meta-analysis has several limitations. The included studies showed a high degree of heterogeneity of patients’ characteristics, most likely attributable to differences in inclusion and exclusion criteria (see Table 1). Some studies included pretreated patients, while other studies included only treatment-naïve patients. Furthermore, there exists no clear definition of pathologic myopia, and so the studies included slightly different patient populations. Some studies did not report on the definition of myopic CNV used in their study, making a comparison even more difficult. Another very relevant exclusion criteria for intravitreal treatment is the history of vitreous surgery. Again, some studies excluded these patients explicitly, while others included them. As the search was limited to publications in English, we might have missed some studies. However, based on visual inspection of funnel plots and analytical methods, we did not observe signs of publication bias. Further, databases were searched for specific keywords, which did not include all treatment options (for example, laser photocoagulation).
Databases were searched for the following keywords: “myopic choroidal neovascularization”.
Another limitation of this study was the different reporting times of the studies e.g., some studies reported on results after one month, three months, or six months. As our sample size would have been too small to compare the exact time points, we had to pool the different follow-up data under the assumption that the different time points were effectively the same. To make the results more comparable, we gave priority to certain time points, i.e., 3 months, then 6 months and 1 month in the early phase, and 24 months, then 12 months, and as a last option, all follow-up time points after 24 months in the late phase. However, the classification of follow-up dates might bias our results. Further, not all studies used the EDTRS charts for visual acuity testing, and we had to calculate the letter score from other scales. Different OCT devices were used for measuring the central retinal thickness in the studies, making comparison difficult. Additionally, few studies reported CRT as an outcome, which weakens the validity of our results.
Another major limitation of this network meta-analysis is the inclusion of non-randomized trials, which could lead to potential bias within each study. In addition, the inclusion of RCTs and observational studies could result in study designs and data collection which are not comparable.
Conclusion
This network meta-analysis shows that intravitreal VEGF inhibitors are the most effective treatment of myopic CNV with few adverse events and a preferred treatment regimen of 1 + PRN.
References
Fredrick DR (2002) Myopia. BMJ (Clinical research ed) 324:1195–1199. https://doi.org/10.1136/bmj.324.7347.1195
Cheung C, Arnold JJ, Holz FG, Park KH, Lai TYY, Larsen M, Mitchell P, Ohno-Matsui K, Chen SJ, Wolf S, Wong TY (2017) Myopic choroidal neovascularization: review, guidance, and consensus statement on management. Ophthalmology 124:1690–1711. https://doi.org/10.1016/j.ophtha.2017.04.028
Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donati G, Lewis H, Lim JI, Menchini U, Miller JW, Mones JM, Potter MJ, Pournaras C, Reaves A, Rosenfeld P, Schachat AP, Schmidt-Erfurth U, Sickenberg M, Singerman LJ, Slakter JS, Strong HA, Virgili G, Williams GA (2003) Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial - VIP report no. 3. Ophthalmology 110:667–673. https://doi.org/10.1016/S0161-6420(02)01998-X
Ruiz-Moreno JM, Montero JA, Arias L, Araiz J, Gomez-Ulla F, Silva R, Piñero DP (2010) Twelve-month outcome after one intravitreal injection of bevacizumab to treat myopic choroidal neovascularization. Retina (Philadelphia, Pa) 30:1609–1615. https://doi.org/10.1097/IAE.0b013e3181e22659
Chen L, Miller JW, Vavvas D, Kim IK (2011) Anti-vascular endothelial growth factor monotherapy versus combination treatment with photodynamic therapy for subfoveal choroidal neovascularization secondary to causes other than age-related macular degeneration. Retin-J Retin Vitr Dis 31:2078–2083. https://doi.org/10.1097/IAE.0b013e3182109074
Howaidy A, Eldaly ZH (2021) Comparison of structural and functional outcome of aflibercept versus ranibizumab in patients with myopic choroidal neovascularization. Eur J Ophthalmol 31(1):211–217. https://doi.org/10.1177/1120672119883590
Kobayashi H, Kobayashi K (2000) Radiotherapy for subfoveal neovascularisation associated with pathological myopia: a pilot study. Br J Ophthalmol 84:761–766. https://doi.org/10.1136/bjo.84.7.761
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784. https://doi.org/10.7326/m14-2385
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed) 372:n71. https://doi.org/10.1136/bmj.n71
Van der Mierden S, Tsaioun K, Bleich A, Leenaars CHC (2019) Software tools for literature screening in systematic reviews in biomedical research. ALTEX 36:508–517. https://doi.org/10.14573/altex.1902131
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
Higgins JPT, Li T, Deeks JJ (eds) (2022). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. Available from www.training.cochrane.org/handbook
Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ (2012) Empirical assessment of within-arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)-EHC141-EF. Agency for Healthcare Research and Quality (US), Rockville (MD)
Ng DS, Kwok AK, Tong JM, Chan CW, Li WW (2015) Factors influencing need for retreatment and long-term visual outcome after intravitreal bevacizumab for myopic choroidal neovascularization. Retina 35:2457–2468. https://doi.org/10.1097/IAE.0000000000000610
Schwarzer G, Carpenter JR, Rücker G (2015) Meta-analysis with R. Springer International Publishing, Switzerland
Balduzzi S, Rücker G, Nikolakopoulou A, Papakonstantinou T, Salanti G, Efthimiou O, Schwarzer G (2023) netmeta: an R Package for network meta-analysis using frequentist methods. J Stat Softw 106(2):1–40. https://doi.org/10.18637/jss.v106.i02
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3:98–110. https://doi.org/10.1002/jrsm.1044
R Core Team (2022) R foundation for statistical computing. Austria, Vienna
Glacet-Bernard A, Benyelles N, Dumas S, Haddad WM, Voigt M, Razavi S, Roquet W, Coscas G, Soubrane G (2007) Photodynamic therapy vs limited macular translocation in the management of subfoveal choroidal neovascularization in pathologic myopia: a two-year study. Am J Ophthalmol 143:68–76. https://doi.org/10.1016/j.ajo.2006.09.041
Hamelin N, Glacet-Bernard A, Brindeau C, Mimoun G, Coscas G, Soubrane G (2002) Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs surgical removal. Am J Ophthalmol 133:530–536. https://doi.org/10.1016/s0002-9394(02)01335-1
Miki A, Honda S, Nagai T, Tsukahara Y, Negi A (2013) Effects of oral bisphosphonates on myopic choroidal neovascularisation over 2 years of follow-up: comparison with anti-VEGF therapy and photodynamic therapy. A pilot study. Br J Ophthalmol 97:770–774. https://doi.org/10.1136/bjophthalmol-2012-303007
Parodi MB, Iacono P, Papayannis A, Sheth S, Bandello F (2010) Laser photocoagulation, photodynamic therapy, and intravitreal bevacizumab for the treatment of juxtafoveal choroidal neovascularization secondary to pathologic myopia. Arch Ophthalmol 128:437–442. https://doi.org/10.1001/archophthalmol.2009.408
Wakabayashi T, Ikeda Y, Gomi F, Tano Y, Hamasaki T (2009) Intravitreal bevacizumab vs sub-tenon triamcinolone acetonide for choroidal neovascularization attributable to pathologic myopia. Am J Ophthalmol 148:591–596
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW (2016) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 123:1351–1359. https://doi.org/10.1016/j.ophtha.2016.02.022
Virgili G, Menchini F (2005) Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst Rev 19:CD004765. https://doi.org/10.1002/14651858.CD004765.pub2
Santini D, Schiavon G, Angeletti S, Vincenzi B, Gasparro S, Grilli C, La Cesa A, Virzí V, Leoni V, Budillon A, Addeo SR, Caraglia M, Dicuonzo G, Tonini G (2006) Last generation of amino-bisphosphonates (N-BPs) and cancer angio-genesis: a new role for these drugs? Recent Pat Anticancer Drug Discov 1:383–396. https://doi.org/10.2174/157489206778776989
Baba T, Kubota-Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S (2010) Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularisation. Br J Ophthalmol 94:864–870
Bandello F, Lanzatta P, Parodi MB, Roman-Pognuz D, Saviano S, Ravalico G (2003) Photodynamic therapy of subfoveal recurrences after laser photocoagulation of extrafoveal choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol 241:567–570. https://doi.org/10.1007/s00417-003-0686-9
Bandello F (2013) Twelve-month efficacy and safety of ranibizumab 0.5 mg (RBZ) versus verteporfin photodynamic therapy (vPDT) in the treatment of visual impairment (VI) due to choroidal neovascularization (CNV) secondary to pathologic myopia (PM). Invest Ophthalmol Vis Sci 54:1247
Brancato R, Menchini U, Pece A, Capoferri C, Avanza P, Radrizzani E (1988) Dye laser photocoagulation of macular subretinal neovascularization in pathological myopia. A randomized study of three different wavelengths. Int Ophthalmol 11:235–238
Chen Y, Sharma T, Li X, Song Y, Chang Q, Lin R, Egger A, Foo A, Gekkieva M, Lai TYY (2019) Ranibizumab versus verteporfin photodynamic therapy in Asian patients with myopic choroidal neovascularization: brilliance, a 12-month, randomized, double-masked study. Retina 39:1985–1994. https://doi.org/10.1097/IAE.0000000000002292
Calvo-González C, Reche-Frutos J, Fernández-Vigo JI, Sáenz-Francés F, Fernández-Pérez C, García-Feijóo J (2017) Long-term outcomes of two different initial dosing regimens of intravitreal ranibizumab used to treat myopic choroidal neovascularization. Ophtalmologica 238:196–204
Cha DM, Kim TW, Heo JW, Woo SJ, Park KH, Yu HG, Chung H (2014) Comparison of 1-year therapeutic effect of ranibizumab and bevacizumab for myopic choroidal neovascularization: a retrospective, multicenter, comparative study. BMC Ophthalmol 14:69. https://doi.org/10.1186/1471-2415-14-69
Chan WM, Lai TY, Wong AL, Liu DT, Lam DS (2007) Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol 91:174–189. https://doi.org/10.1136/bjo.2006.103606
Chen L, Miller JW, Vavvas D, Kim IK (2011) Anti-vascular endothelial growth factor monotherapy versus combination treatment with photodynamic therapy for subfoveal choroidal neovascularization secondary to causes other than age-related macular degeneration. Retina 31:2078–2083. https://doi.org/10.1097/IAE.0b013e3182109074
Chen C, Yan M, Huang Z, Song Y-P (2020) The evaluation of a two-year outcome of intravitreal conbercept versus ranibizumab for pathological myopic choroidal neovascularization. Curr Eye Res 45:1415–1421. https://doi.org/10.1080/02713683.2020.1742357
Costa RA, Williams GA (2006) Two-fold illumination scheme for photodynamic therapy study group. Twofold illumination photodynamic therapy scheme for subfoveal choroidal neovascularization in pathologic myopia: results from a randomized pilot study. Retina 26:757–764. https://doi.org/10.1097/01.iae.0000244260.52901.2e
Dethorey G, Leveziel N, Glacet-Bernard A, Lalloum F, Hay A, Tahiri R, Champion E, Souied EH (2010) Efficacy of ranibizumab versus PDT in myopic choroidal neovascularisation. Invest Ophthalmol Vis Sci 51:2204
El Habbak A, El Nagar M, Tawfik T, El Zaher MA, El Shiwy H, Howaidy A, Falougy A (2016) Comparison of intravitreal aflibercept injection versus intravitreal ranibizumab injection in patients with myopic choroidal neovascularization. Ophthalmologica J Int d’ophtalmologie Int J Ophthalmol Zeitschrift fur Augenheilkunde 236:38–39
Erden B, Bölükbaşı S, Baş E, Çakır A (2019) Comparison of intravitreal aflibercept and ranibizumab for treatment of myopic choroidal neovascularization: one-year results-a retrospective, comparative study. J Ophthalmol 2019:8639243. https://doi.org/10.1155/2019/8639243
Farinha C, Baltar AS, Nunes SG, Franqueira NF, Figueira JP, Pires IA, Cachulo ML, Silva RM (2013) Choroidal thickness after treatment for myopic choroidal neovascularization. Eur J Ophthalmol 23:887–898. https://doi.org/10.5301/ejo.5000323
Fernández RD, Govetto A, Alves Perez MT, Lorente R (2013) Ranibizumab versus bevacizumab in the treatment of subfoveal choroidal neovascular membrane secondary to pathologic myopia. Invest Ophthalmol Vis Sci 54:4936
Wang E, Chen Y (2013) Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta-analysis. Retina 33(7):1375–92. https://doi.org/10.1097/IAE.0b013e31827d260a
Freitas-da-Costa P, Pinheiro-Costa J, Carvalho B, Falcão M, Brandão E, Falcão-Reis F, Carneiro  (2014) Anti-VEGF therapy in myopic choroidal neovascularization: long-term results. Ophtalmologica 232:57–63. https://doi.org/10.1159/000360307
Gharbiya M, Giustolisi R, Allievi F, Fantozzi N, Mazzeo L, Scavella V, Gabrieli CB (2010) Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab–a randomized controlled trial. Am J Ophthalmol 149:458–464. https://doi.org/10.1016/j.ajo.2009.10.010
Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, Moriyama M, Hara W, Yoshida T, Tokoro T, Mochizuki M (2008) Photodynamic therapy with verteporfin for choroidal neovascularization of pathologic myopia in Japanese patients: comparison with nontreated controls. Am J Ophthalmol 145:518–526
Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, Moriyama M, Hayashi W, Yoshida T, Tokoro T, Mochizuki M (2009) Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol 148:396–408. https://doi.org/10.1016/j.ajo.2009.03.026
Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Cascavilla ML, Bandello F (2012) Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina 32:1539–1546. https://doi.org/10.1097/IAE.0b013e31826956b7
Iacono P, Battaglia Parodi M, Selvi F, Parravano MC, Chiaravalloti A, Varano M, Bandello F (2017) Factors influencing visual acuity in patients receiving anti-vascular endothelial growth factor for myopic choroidal neovascularization. Retina 37:1931–1941. https://doi.org/10.1097/IAE.0000000000001436
Ikuno Y, Nagai Y, Matsuda S, Arisawa A, Sho K, Oshita T, Takahashi K, Uchihori Y, Gomi F (2010) Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol 149:140–146. https://doi.org/10.1016/j.ajo.2009.08.008
Introini U, Casalino G, Querques G, Gimeno AT, Scotti F, Bandello F (2012) Spectral-domain OCT in anti-VEGF treatment of myopic choroidal neovascularization. Eye 26:976–982
Kang EC, Seo JG, Kim BR, Koh HJ (2017) Clinical outcomes of intravitreal bevacizumab versus photodynamic therapy with or without bevacizumab for myopic choroidal neovascularization: a 7-year follow-up study. Retina 37:1775–1783. https://doi.org/10.1097/IAE.0000000000001421
Korol A, Kustryn T, Zadorozhnyy O, Pasyechnikova N, Kozak I (2020) Comparison of efficacy of intravitreal ranibizumab and aflibercept in eyes with myopic choroidal neovascularization: 24-month follow-up. J Ocul Pharmacol Ther 36:122–125
Lai TY, Luk FO, Lee GK, Lam DS (2012) Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye 26:1004–1011. https://doi.org/10.1038/eye.2012.97
Li S, Ding X, Sun L, Zhao X, Zhang A, Lyu C, Liu B, Zhang J, Jin C, Lu L (2019) Two different initial treatment regimens of ranibizumab in myopic choroidal neovascularization: 12-month results from a randomized controlled study. Clin Exp Ophthalmol 47:250–258. https://doi.org/10.1111/ceo.13424
Matsuo M, Honda S, Matsumiya W, Kusuhara S, Tsukahara Y, Negi A (2012) Comparison between anti-vascular endothelial growth factor therapy and photodynamic therapy for myopic choroidal neovascularization. Eur J Ophthalmol 22:210–215
Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik J-F, Vitti R, Li T, Stemper B, Asmus F, Zeitz O, Ishibashi T (2015) Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology 122:1220–1227
Niwa Y, Sawada O, Miyake T, Kakinoki M, Sawada T, Kawamura H, Ohji M (2012) Comparison between one injection and three monthly injections of intravitreal bevacizumab for myopic choroidal neovascularization. Ophthalmic Res 47:135–140. https://doi.org/10.1159/000330499
Pal B, Degli Esposti S, DeZaeytijd J, Rahman W, Adatia F, Hamilton RD, Tufail A (2010) Visual outcome in myopic choroidal neovascularisation following anti-VEGF (bevacizumab or ranibizumab) therapy compared to photodynamic treatment (PDT) or observation. Invest Ophthalmol Vis Sci 51:2197
Parravano M, Ricci F, Oddone F, Missiroli F, De Felici C, Varano M (2014) Long-term functional and morphologic retinal changes after ranibizumab and photodynamic therapy in myopic choroidal neovascularization. Retina 34(10):2053–62. https://doi.org/10.1097/IAE.0000000000000201
Pece A, Milani P, Monteleone C (2015) A randomized trial of intravitreal bevacizumab vs. ranibizumab for myopic CNV. Graefes Arch Clin Exp Ophthalmol 253:1867–1872
Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, Wong TY, Silva R, Pilz S, Gekkieva M, RADIANCE Study Group (2014) RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 121:682–692. https://doi.org/10.1016/j.ophtha.2013.10.023
Rinaldi M, Semeraro F, Chiosi F, Russo A, Romano MR, Savastano MC, dell’Omo R, Costagliola C (2017) Reduced-fluence verteporfin photodynamic therapy plus ranibizumab for choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol 255:529–539
Rishi P, Rishi E, Venkataraman A, Gopal L, Sharma T, Bhende M, Ratra D, Sen PR, Sen P (2011) Photodynamic monotherapy or combination treatment with intravitreal triamcinolone acetonide, bevacizumab or ranibizumab for choroidal neovascularization associated with pathological myopia. Indian J Ophthalmol 59:242–246
Rishi P, Rishi E, Bhende M, Agarwal V, Vyas CH, Valiveti M, Bhende P, Rao C, Susvar P, Sen P, Raman R, Khetan V, Murali V, Ratra D, Sharma T (2016) Comparison of photodynamic therapy, ranibizumab/bevacizumab or combination in the treatment of myopic choroidal neovascularisation: a 9-year-study from a single centre. Br J Ophthalmol 100:1337–1340
Ruiz-Moreno JM, López-Gálvez MI, Donate J, Gomez-Ulla F, García-Arumí J, García-Layana A, Sellés I, Reche J, Montero JA, Pazos B, Zapata MA, Pastor JC (2011) Myopic choroidal neovascularization. Ophthalmology 118(12):2521–3. https://doi.org/10.1016/j.ophtha.2011.07.029
Ruiz-Moreno JM, Montero JA, Amat-Peral P (2011) Myopic choroidal neovascularization treated by intravitreal bevacizumab: comparison of two different initial doses. Graefes Arch Clin Exp Ophthalmol 249:595–599. https://doi.org/10.1007/s00417-010-1599-z
Ruiz-Moreno JM, Montero JA, Arias L, Araiz J, Gomez-Ulla F, Silva RDPP (2012) Three versus one intravitreal bevacizumab injections as initial protocol to treat myopic choroidal neovascularization. Acta Ophthalmol 90:e82-83. https://doi.org/10.1111/j.1755-3768.2010.02070.x
Ruiz-Moreno JM, Arias L, Montero JA, Carneiro Â, Silva R (2013) Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol 97:1447–1450
Ruiz-Moreno JM, López-Gálvez MI, Montero JA, Pastor JC (2013) Intravitreal bevacizumab in myopic neovascular membranes: 24-month results. Ophtalmology 120:1510–1511. https://doi.org/10.1016/j.ophtha.2013.03.006
Ruiz-Moreno JM, Montero JA, Araiz J, Arias L, García-Layana J, Carneiro Â, Figueroa MS, Silva R (2015) Intravitreal anti-vascular endothelial growth factor therapy for choroidal neovascularization secondary to pathologic myopia: six years outcome. Retina 35:2450–2456. https://doi.org/10.1097/IAE.0000000000000632
Saviano S, Piermarocchi R, Leon EP, Mangogna A, Zanei A, Cavarzeran F, Tognetto D (2014) Combined therapy with bevacizumab and photodynamic therapy for myopic choroidal neovascularization: a one-year follow-up controlled study. Int Ophthalmol 7:335–339
Sayanagi K, Uematsu S, Hara C, Wakabayashi T, Fukushima Y, Sato S, Ikuno Y, Nishida K (2019) Effect of intravitreal injection of aflibercept or ranibizumab on chorioretinal atrophy in myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 257:749
Siu-Chun D, Kwok AKH, Tong JMK, Chan CWN, Li WWT (2015) Factors influencing need for retreatment and long-term visual outcome after intravitreal bevacizumab for myopic choroidal neovascularization. Retina (Philadelphia, Pa) 35:2457–2468. https://doi.org/10.1097/IAE.0000000000000610
Verteporfin in Photodynamic Therapy (VIP) Study Group, Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, Lim JI, Menchini U, Miller JW, Mones JM, Potter MJ, Pournaras C, Reaves A, Rosenfeld P, Schachat AP, Schmidt-Erfurth U, Sickenberg M, Singerman LJ, Slakter JS, Strong HA, Virgili G, Williams GA (2003) Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial–VIP report no.3. Ophthalmology 110:667–673. https://doi.org/10.1016/s0161-6420(02)01998-x
Verteporfin in Photodynamic Therapy (VIP) Study Group (2001) Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial-VIP report no 1. Ophthalmology 108:841–852. https://doi.org/10.1016/s0161-6420(01)00544-9
Voykov B, Gelisken F, Inhoffen W, Voelker M, Bartz-Schmidt KU, Ziemssen F (2010) Bevacizumab for choroidal neovascularization secondary to pathologic myopia: is there a decline of the treatment efficacy after 2 years? Graefes Arch Clin Exp Ophthalmol 248:543–550. https://doi.org/10.1007/s00417-009-1285-1
Wakabayashi T, Ikuno Y, Gomi F (2011) Different dosing of intravitreal bevacizumab for choroidal neovascularization because of pathologic myopia. Retina (Philadelphia, Pa) 31:880–886. https://doi.org/10.1097/IAE.0b013e3181f2a293
Wang JK, Huang TL, Chang PY, Chen YT, Chang CW, Chen FT, Hsu YR, Chen YJ (2018) Intravitreal aflibercept versus bevacizumab for treatment of myopic choroidal neovascularization. Sci Rep 8:14389. https://doi.org/10.1038/s41598-018-32761-z
Woronkowicz M, Lightman S, Hamilton R, Zagora S, Tomkins-Netzer O (2018) Comparison of anatomical and functional outcomes of treatment with bevacizumab and ranibizumab injections in eyes with myopic choroidal neovascularization (mCNV). Invest Ophthalmol Vis Sci 59:4745
Yoon JU, Byun YJ, Koh HJ (2010) Intravitreal anti-VEGF versus photodynamic therapy with verteporfin for treatment of myopic choroidal neovascularization. Retina 30:418–424. https://doi.org/10.1097/IAE.0b013e3181bd2fe4
Yoon JU, Kim YM, Lee SJ, Byun YJ, Koh HJ (2012) Prognostic factors for visual outcome after intravitreal anti-VEGF injection for naive myopic choroidal neovascularization. Retina 32:949–955. https://doi.org/10.1097/IAE.0b013e318227a9ef
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Glachs, L., Embacher, S., Berghold, A. et al. Treatment of myopic choroidal neovascularization: a network meta-analysis and review. Graefes Arch Clin Exp Ophthalmol 262, 1693–1722 (2024). https://doi.org/10.1007/s00417-023-06271-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06271-2