Abstract
Background
The association of obstructive sleep apnea (OSA) with development of eye diseases is unclear. This current systematic review and meta-analysis attempts to summarize and analyze associations between OSA and ocular disorders in the literature.
Methods
PubMed, EMBASE, Google Scholar, Web Of Science, and Scopus databases were searched from 1901 to July 2022 in accordance with the Preferred Reporting in Systematic Review & Meta-Analysis (PRISMA). Our primary outcome assessed the association between OSA and the odds of developing floppy eyelid syndrome (FES), glaucoma, non-arteritic anterior ischemic optic neuropathy (NAION), retinal vein occlusion (RVO), keratoconus (KC), idiopathic intracranial hypertension (IIH), age-related macular degeneration (AMD), and central serous chorioretinopathy (CSR) through odds ratio calculated at the 95% confidence interval.
Results
Forty-nine studies were included for systematic review and meta-analysis. The pooled OR estimate was highest for NAION [3.98 (95% CI 2.38, 6.66)], followed by FES [3.68 (95% CI 2.18, 6.20)], RVO [2.71(95% CI 1.83, 4.00)], CSR [2.28 (95% CI 0.65, 7.97)], KC [1.87 (95% CI 1.16, 2.99)], glaucoma [1.49 (95% CI 1.16, 1.91)], IIH [1.29 (95% CI 0.33, 5.01)], and AMD [0.92 [95% CI 0.24, 3.58] All observed associations were significant (p < 0.001) aside from IIH and AMD.
Conclusion
OSA is significantly associated with NAION, FES, RVO, CSR, KC, and glaucoma. Clinicians should be informed of these associations so early recognition, diagnosis, and treatment of eye disorders can be addressed in at-risk groups, and early referral to ophthalmic services is made to prevent vision disturbances. Similarly, ophthalmologists seeing patients with any of these conditions should consider screening and referring patients for assessment of possible OSA.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder with disturbed breathing resulting from repetitive total or partial collapse of the upper respiratory tract during sleep. Breathing cessation generally lasts for 10 to 30 s causing swings between hypoxia and reperfusion, which if chronic, ultimately leads to pulmonary hypertension [1]. As the most common sleep disorder, its prevalence ranges from 22 to 24% in men, 9 to 17% in women, and 6% in adolescents. OSA is now considered a global public health issue as incidence climbs and its associated risks with other diseases and mortality grow [2]. OSA is a well-known major risk factor for many cardiovascular and metabolic health issues [3], but is also significantly associated with ophthalmic disorders including floppy eyelid syndrome (FES) [4,5,6,7,8,9], papilledema [10], non-arteritic anterior ischemic optic neuropathy (NAION), [11,12,13,14,15,16,17,18,19,20] keratoconus (KC), [21,22,23,24,25] central serous chorioretinopathy (CSR) [26,27,28,29], retinal vein occlusion (RVO) [30,31,32,33], glaucoma [5, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], age-related macular degeneration (AMD) [51, 52], and idiopathic intracranial hypertension (IIH) [14, 53,54,55]. Many hypotheses for these associations have been proposed, but intermittent hypoxia, excessive sympathetic stimulation, oxidative stress, and damaging effects of endothelin-1 are mechanisms thought to be involved with the pathophysiology [56].
These ocular morbidities associated with OSA have resulted in research to better understand underlying mechanisms, specific populations at risk, and prediction of morbidity. A better understanding surrounding these could lead to referral of at-risk groups to ophthalmic services shortly after diagnosis to preserve sight and prevent significant morbidity, and conversely, screening and referral pathways for ophthalmologists may be established for patients at-risk for OSA with common ocular manifestations.
Before these interventions can be widely acknowledged as critical for preserving eye health, a consensus about the associations of ocular disorders with OSA needs to be met. One systematic review and meta-analysis in the past has been conducted; however, an influx of new studies and the exclusion of other potentially important conditions like KC and IIH leaves room for conjecture [57]. This systematic review and meta-analysis attempts to assist in this process by summarizing and analyzing associations between OSA and ocular disorders in the current literature.
Methods
Methodology statement
This review adhered to the Preferred Reporting in Systematic Review & Meta-Analysis (PRISMA) [58] guidelines and was listed on the PROSPERO, International Prospective Register of Systematic Review (CRD42022314672). There were no study restrictions imposed on different populations, races, ethnicity, origin, and language.
Literature search
Online electronic databases (PubMed, EMBASE, Google Scholar, Web Of Science, and Scopus) were searched till July 2022 using the search terms: (“Obstructive sleep apnea/hypopnea syndrome” or “OSAHS” or “sleep apnea syndrome” or “OSA” or “obstructive sleep apnea”) and the ophthalmologic disorders individually (“floppy eyelid syndrome,” “glaucoma,” “non-arteritic anterior ischemic optic neuropathy,” “retinal vein occlusion,” “keratoconus,” “idiopathic intracranial hypertension,” “age related macular degeneration,” “central serous chorioretinopathy”). The search terms were used in different combinations. No age, gender, and population filters were imposed. Manual search of included studies and previous reviews’ references was performed.
Inclusion and exclusion criteria
The established inclusion criteria were as follows: (1) all published studies evaluating the association between OSA and association of specific ophthalmologic disorders, (2) all full-text studies including randomized control trials, original research articles, descriptive and analytic studies (cohort or case-control), (3) studies providing sufficient data to calculate odds ratio (OR) and 95% confidence interval (CI), and (4) studies published in English and conducted on human participants. There was no limit on the population group in terms of age, sex, ethnicity, or co-morbidities.
The exclusion criteria were as follows: (1) studies not measuring conclusive risk estimates, (2) studies without available full-text, (3) poster or scientific presentations, (4) reviews, meta-analysis, opinion articles, surveys, letter to editor, short communications, case reports, case series, abstracts, commentaries, and book chapters.
Outcome measures
The primary outcome measures assessed the strength of association between OSA and associated ophthalmologic comorbidities like floppy eyelid syndrome, glaucoma, non-arteritic anterior ischemic optic neuropathy, retinal vein occlusion, keratoconus, idiopathic intracranial hypertension, age-related macular degeneration, and central serous chorioretinopathy, in terms of odds ratio calculated at 95% confidence interval (CI).
Data extraction and quality assessment
Two authors independently (IS and GB) screened all titles and abstracts against predefined inclusion and exclusion criteria, and any disparity in either selecting eligible studies or assessing findings between the two reviewers was resolved through consultation with a third reviewer (SS). The complete texts of the studies were then obtained and read in full to fulfill the final inclusion. Any differences in articles selected by the two were discussed to reach a decision regarding inclusion. The reference lists of screened articles were also reviewed for any missed literature. The information recorded for each selected study included the name of the first author, publication year, study design, participant selection, total number of cases and controls, methods for the diagnosis of OSA, systemic disease prevalence (hypertension, diabetes, and body mass index), presence of adjustment for covariates, CPAP status, and the author’s remarks/conclusions.
Statistical analysis
The meta-analysis was performed using review manager (RevMan version 5.4, Cochrane collaboration, Oxford, UK). Summary of OR estimates from each study was calculated by a random-effects Mantel- Haenszel method, and results presented as an odds ratio (OR) with 95% confidence intervals (CIs). Statistical difference between the groups was considered to be present if the pooled 95% CI did not include 1 for the respective OR. For each factor analyzed, a forest plot showing the respective odds ratios or standardized mean differences with their corresponding 95% confidence interval for each study and the pooled data were generated. The test of overall effect was assessed using the Z statistics on RevMan v5.4 with statistical significance set at p < 0.05.
Heterogeneity (inconsistency) between studies was evaluated using the Cochrane Q (Chi2 test) and I2 statistics in RevMan v5.4. Estimates of degree of heterogeneity using I2 were made by setting 25%, 50%, or 75% as limits for low, moderate, or high heterogeneity, respectively. Random-effects model with weighting of the studies was used when there was heterogeneity between studies with I2 values of over 50%. All p values were two-tailed and considered statistically significant if less than 0.05.
Results
Study selection
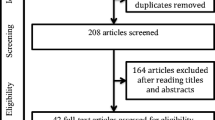
A total of 519 potentially eligible records were extracted in the initial data retrieval process. During the screening, 245 records were omitted following duplication screening, and 22 were eliminated for not being in English. Of the 252 records assessed for eligibility, 183 studies were excluded for not meeting the inclusion criteria. Forty-nine studies were finally included for quantitative assessment and meta-analysis. Mild to moderate heterogeneity of included studies was observed based on I2 statistics less than 50%. Characteristics of the included studies are detailed in Table 1. The process used to search and identify studies is illustrated in Fig. 1. Basic cohort characteristics are listed in Table 2. The average cohort percentages for diabetes and hypertension were 24.04% and 38.12%, respectively, from 11 and nine studies reporting on these statistics in OSA patients. The average BMI was 30.4, reported from 13 studies. Just two studies reported their participants using CPAP, 30 studies identified diagnosis of OSA at the time of the study and were deemed CPAP-naive, while the remaining 17 used retrospective data and reported no use of CPAP.
Glaucoma
A total of 21 studies were included (8 case control, 6 cross-sectional, 4 cohort, 2 retrospective chart order review, and 1 database study). The overall pooled OR estimate was assessed as 1.49 (95% CI 1.16, 1.91). For cross-sectional studies, the aggregate OR estimate was 3.74 (95% CI 1.48, 9.48), much higher compared to prospective studies where pooled OR estimate was 1.50 (95% CI 0.93, 2.45) (Fig. 2).
Floppy eyelid syndrome
Of the six studies included for analysis, three were cross-sectional studies and three case-control studies. While the overall pooled OR was 3.68 (95% CI 2.18, 6.20), the aggregate OR estimate for cross-sectional studies was 3.88 (95% CI 1.55, 9.69) and prospective studies was 3.69 (95% CI 1.66, 8.23) (Fig. 3).
Central serous chorioretinopathy
One case-control study and one population-based study were included for analysis. The pooled OR was assessed to be 2.28 (95% CI 0.65, 7.97) (Fig. 4).
Keratoconus
The study included four case-control studies and one database study reporting OSA incidence in keratoconus patient population. A total of 1838 OSA cases were noted among 16,922 pooled keratoconus cases, with pooled OR estimate analyzed as 1.87 (95% CI 1.16, 2.99) (Fig. 5).
Non-arteritic anterior ischemic optic neuropathy
A total of ten studies were included, of which six were case-control studies, three were retrospective cohort studies, and one database study which reported risk of OSA in NAION population. Pooled OR estimates observed was 3.98 (95% CI 2.38, 6.66) (Fig. 6).
Retinal vein occlusion
Of the four studies included, three were case-control studies reporting prevalence of OSA in RVO cases, and one was a population-based study reporting prevalence of RVO in OSA group. A pooled OR of 2.71 (95% CI 1.83, 4.00) was analyzed (Fig. 7).
Idiopathic intracranial hypertension
Four studies investigated OSA in IIH patients were included. One was a prospective cohort, one was case-control study, and the other two were database record studies. Overall pooled OR was estimated as 1.29 (95% CI 0.33, 5.01) (Fig. 8).
Age-related macular degeneration
Two cohort studies reported incidence of OSA in an AMD population. A pooled OR of 0.92 [95% CI 0.24, 3.58] was concluded (Fig. 9).
Discussion
The current systematic review and meta-analysis analyzed the incidence of OSA with common ocular disorders, and our analysis revealed significantly raised odds ratios for OSA with NAION, followed by FES, RVO, keratoconus, CSR, and glaucoma. No raised OR was observed with AMD. Aside from two studies, participants were either CPAP-naïve or the information was not disclosed. Therefore, the results of this study suggest people with OSA are at risk of serious eye disorders and would benefit from screening programs and follow-up to minimize their risk of irreversible vision loss and morbidity. Conversely, as OSA is an underdiagnosed disorder [59, 60], individuals presenting with ocular disorders should also be screened for OSA for holistic management.
This study found a 3.98 times greater risk for NAION with OSA, which is higher than a previous 2016 meta-analysis by Huon et al. where OSA conferred a 3.126 times greater risk for neuropathy [57]. NAION presents as a sudden, painless, usually unilateral, vision loss which commonly occurs upon awakening from sleep. It is thought to manifest from a microvascular infarction of the optic nerve from nocturnal hypoxemia of the short posterior ciliary arteries, which in this study shown is very likely to be precipitated by OSA [61]. Although the association of NAION and OSA has been discussed in various studies [11,12,13,14,15,16,17,18,19,20], the prevalence of OSA varies. Our meta-analysis included ten case-control studies where reported prevalence ranged from 0.06 to 94.73%. Of all studies, Bandi et al. [15] recorded the highest prevalence of NAION (18/19) OSA cases. The addition of Bandi et al., which was not in a previous meta-analysis, likely led to the higher OR reported by this study. The ranges of prevalence across studies may be due to heterogeneity in recruitment strategies for OSA, where Bandi et al. [15] used polysomnography and Li et al. [12] used the Sleep Apnea scale of the Sleep Disorders Questionnaire. Ethnic differences between Iranian and Caucasian populations also likely played a role in these differences. Even so, heterogeneity was high (I2 = 91%) and so NAION is likely raised as a product of OSA beyond a reasonable doubt.
Our meta-analysis showed OSA was associated with 3.68 times greater risk of developing FES which is slightly higher than a previous meta-analysis (OR = 3.126) [57]. FES is an ocular condition characterized by ease of eyelid eversion with slight upward traction and tissue that has become flaccid from loss of elasticity [62]. This meta-analysis identified prevalence ranges from 25.84% reported by Chambe et al. [8], to as high as 64.57% reported by Acar et al. [9] which upon further review, can be attributed to grading differences. While Chambe et al. [8] had split eyelid syndromes into FES and lax eyelid syndrome (LES), the latter being a milder version of the former, Acar et al. [9] graded FES. In considering LES, prevalence in OSA rose to 49.4% generally, and 75% in severe OSA, which ended up matching Acar’s finding of 74.6% for severe OSA. This likely unfortunately underestimates the OR in this analysis even though there was still a significantly raised odds ratio and reasonably low heterogeneity among the included studies (I2 = 29%).
Four studies were included in our analysis and the presence of OSA resulted in 2.71-fold increased odds of developing RVO, which was much higher Chou et al.’s reported OR of 1.94 [30, 57]. This is the first meta-analysis performed on RVO as the previous study had insufficient studies to analyze an association [57]. The risk of RVO may be increased by OSA through hypoxic insult to blood flow autoregulation mechanisms and the microvasculature, weakening the resilience of vessels to sudden occlusions. Considering the rise in risk with the addition of newer studies, more cohort studies should analyze this association to ensure consistency in risk and prevalence as RVO is an ophthalmic emergency, and elucidating risk in OSA patients can better educate practitioners.
KC, a non-inflammatory ectatic corneal condition characterized by progressive steepening and thinning of the cornea, was associated with higher risk of OSA in this study (OR = 1.87). It is suggested that higher activity of proteolytic enzyme matrix metalloproteinases (MMPs) degrade extracellular matrix in hypoxic response to stress or injury contributes to this association, as in KC patients MMP-9 in tears are increased and likely contribute to corneal thinning [63]. Our study included four case controls and a database study where the prevalence of keratoconus among OSA patients varied from 7.53% (11/146) reported by Gencer et al. [23] to 26.67% (4/15) reported by Pihlblad and Schaefer [22]. Our pooled OR estimate is close to that of the meta-analysis performed by Pellegrini M et al. [63] where the odds of OSA in KC patients was 1.8 times higher as compared to controls (OR = 1.841).
OSA has previously been associated with CSR, a condition of the eye where the neuro-sensory retina in the macular region is detached by the collection of serous fluid, resulting in mild loss of visual acuity, decreased contrast sensitivity, and visual and color distortion. Likely, increased circulating epinephrine and norepinephrine levels in OSA patients increase sympathetic tone, which causes endothelial dysfunction in the blood-retinal barrier and retinal serous fluid accumulation [64]. Our analysis included two studies with variance in prevalence ranging from 0.17 [29] to as high as 7.65% [28] and a pooled OR being 2.28. Our pooled estimate was almost in line with the study conducted by Wu et al. [65], which included six studies for systematic review and meta-analysis which showed CSR patients had 1.56 increased odds of having OSA than controls. This is considerably lower than the previous meta-analysis by Hou et al. although they only included two studies [26, 27].
Our meta-analysis included a total of 21 studies on the association between OSA and primary open-angle glaucoma, which was the largest study pool of all analyses. The meta-analysis showed 1.49 times higher risk for developing glaucoma in OSA, and variance in incidence ranged from 41.67% by Bilgin et al. [49] 41.67% (10/24), to 27.36% in a case-control study by Onen et al. [34] of 212 cases. By predisposing the optic nerve head to ischemia, OSA inflicts repeated hypoxic events, hemodynamic changes to retinal blood vessels, oxidative stress, mitochondrial dysregulation, and inflammation, which contribute to nerve fiber dysfunction and degeneration in glaucoma. It is possible the association of OSA with glaucoma may be partially confounded by other diseases resulting in poor perfusion, for example, obesity, hypertension, and diabetes [29].
While OSA has also been associated with AMD in the past [51], our pooled estimate from two included studies confers no raised odds of AMD in an OSA cohort. Recent research suggests hypoxia and oxidative stress from OSA trigger inflammatory processes which play roles in AMD pathogenesis [52]. In addition, OSA’s association with non-responsiveness to anti-VEGF for AMD treatments has perpetuated the belief it has some influence on the disease [51]; however, this meta-analysis proposes otherwise. While there are many overlapping factors that may hinder a true association, including obesity and older age [52], it should be considered only two studies were available for analysis. Therefore, future research should investigate the certainty of our findings further.
This study collectively examined associations between OSA and a range of ocular conditions and analyzed the association with IIH for the first time. Overall pooled OR from four included studies was 1.29, suggesting slightly raised odds of sleep apnoea with IIH (p < 0.001). OSA is known to increase intracranial pressure through hypercapnia, hypoxia, and cerebral vasodilation that increases intracranial volume [14]. A paper has suggested use of CPAP can reverse IIH in those with contiguous OSA, supporting this link and providing a realistic solution if IIH is coincident [14]. As some studies did not control obesity, it is possible this is a confounder for this finding considering body weight can limit chest expansion and cause hypoxia and hypercapnia like OSA [14].
Our findings highlight OSA is associated with a raised OR for a range of serious and debilitating eye disorders, and an increase in studies available for meta-analysis has allowed for these relationships to be better elucidated. Considering retinal tissue has one of the highest oxygen demands in the body, it is clearly liable to hypoxic injury by nocturnal events in OSA patients [29]. Thus, regular screening of OSA patients for ophthalmologic complications should be encouraged to protect patients from irreversible vision loss.
Despite these contributions, some limitations should be addressed. First, meta-regression analysis of various confounding factors was beyond the scope of this study due to age, sex, ethnicity, and disease duration that can deter the odds ratio. It did not help that confounding variables like hypertension, diabetes, body mass index, and other systemic diseases prevalent in both OSA and eye diseases were not reported by studies in OSA participants with eye diseases. Despite this, it was observed most studies had multivariate cox regression models, propensity-matched participants, and backward stepwise analyses in place to minimize confounding, even if these were not explicitly listed as characteristics of OSA participants with eye diseases. Future studies could be improved by ensuring multivariate models were in place across all studies to ensure a finding is independent of other confounders. Second, the severity of OSA in various patients was not often evaluated, which could have otherwise affected the odds of ocular morbidities, and CPAP treatment status was not explicitly listed in most studies. While it was clear in prospective studies that participants were CPAP-naïve, retrospective studies using ICD codes did not detail whether participants were offered/on therapy, making it possible endothelial dysfunction caused by OSA could be reversed and minimized as a cause for ocular disease. Even so, OSA preceded the onset of eye diseases in these studies, and retrospective study designs were most likely to rigorously control for confounding factors. This makes it likely OSA contributed to the event nonetheless, although the effect may be augmented if some populations were treated with CPAP. Third, the vast variance in prevalence of our pooled studies is due to heterogeneity of study designs between studies and may impact generalizability. Because the database studies had an enormous sample size to pool estimates while case-control studies had limited samples in comparison, this likely exacerbates the effect. Also, the sample size for some ocular morbidities varies in comparison to others due to varied prevalence rates.
Conclusion
This systematic review and meta-analysis study showed significant associations between OSA and NAION, FES, RVO, KC, CSR, and glaucoma, but no association with AMD. Considerable heterogeneity throughout analyses may impact the generalizability of our findings. Vision specialists and referring physicians should be aware of the risks associated with OSA, and various ocular morbidities and patients referred for early recognition, diagnosis, and treatment. Conversely, ophthalmologists seeing patients with any of these conditions should consider referring patients for assessment of possible OSA if that has not already occurred.
References
Goyal M, Johnson J (2017) Obstructive sleep apnea diagnosis and management. Mo Med 114(2):120–124
Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis 7(8):1311–1322. https://doi.org/10.3978/j.issn.2072-1439.2015.06.11
Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. Lancet 383(9918):736–747. https://doi.org/10.1016/S0140-6736(13)60734-60735
Karger RA, White WA, Park WC et al (2006) Prevalence of floppy eyelid syndrome in obstructive sleep apnea-hypopnea syndrome. Ophthalmology 113(9):1669–1674. https://doi.org/10.1016/j.ophtha.2006.02.053
Kadyan A, Asghar J, Dowson L et al (2010) Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye (Lond) 24(5):843–850. https://doi.org/10.1038/eye.2009.212
Ezra DG, Beaconsfield M, Sira M et al (2010) The associations of floppy eyelid syndrome: a case control study. Ophthalmology 117(4):831–838. https://doi.org/10.1016/j.ophtha.2009.09.029
Beis PG, Brozou CG, Gourgoulianis KI, Pastaka C, Chatzoulis DZ, Tsironi EE (2012) The floppy eyelid syndrome: evaluating lid laxity and its correlation to sleep apnea syndrome and body mass index. ISRN Ophthalmol 650892. https://doi.org/10.5402/2012/650892
Chambe J, Laib S, Hubbard J et al (2012) Floppy eyelid syndrome is associated with obstructive sleep apnoea: a prospective study on 127 patients. J Sleep Res 21(3):308–315. https://doi.org/10.1111/j.1365-2869.2011.00968.x
Acar M, Firat H, Acar U et al (2013) Ocular surface assessment in patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath 17(2):583–588. https://doi.org/10.1007/s11325-012-0724-0
Purvin VA, Kawasaki A, Yee RD (2000) Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol 118(12):1626–1630. https://doi.org/10.1001/archopht.118.12.1626
Mojon DS, Hedges TR 3rd, Ehrenberg B et al (2002) Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol 120(5):601–605. https://doi.org/10.1001/archopht.120.5.601
Palombi K, Renard E, Levy P et al (2006) Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol 90:879–882. https://doi.org/10.1136/bjo.2005.087452
Li J, McGwin G Jr, Vaphiades MS et al (2007) Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the Sleep Apnea scale of the Sleep Disorders Questionnaire. Br J Ophthalmol 91(11):1524–1527. https://doi.org/10.1136/bjo.2006.113803
Stein JD, Kim DS, Mundy KM et al (2001) The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol 152(6):989–998. https://doi.org/10.1016/j.ajo.2011.04.030e983
Arda H, Birer S, Aksu M et al (2013) Obstructive sleep apnoea prevalence in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 97(2):206–209. https://doi.org/10.1136/bjophthalmol-2012-302598
Bilgin G, Koban Y, Arnold AC (2013) Nonarteritic anterior ischemic optic neuropathy and obstructive sleep apnea. J Neuroophthalmol 33(3):232–234. https://doi.org/10.1097/WNO.0b013e31828eecbd
GhalehBandi MF, Naserbakht M, Tabasi A et al (2015) Obstructive sleep apnea syndrome and non-arteritic anterior ischemic optic neuropathy: a case control study. Med J Islam Repub Iran 29:300
Cestari DM, Gaier ED, Bouzika P et al (2016) Demographic, systemic, and ocular factors associated with nonarteritic anterior ischemic optic neuropathy. Ophthalmology 123:2446–2455. https://doi.org/10.1016/j.ophtha.2016.08.017
Sun MH, Lee CY, Liao YJ et al (2019) Nonarteritic anterior ischaemic optic neuropathy and its association with obstructive sleep apnoea: a health insurance database study. Acta Ophthalmol 97(1):64–70. https://doi.org/10.1111/aos.13832
Yang HK, Park SJ, Byun SJ et al (2019) Obstructive sleep apnoea and increased risk of non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 103(8):1123–1128. https://doi.org/10.1136/bjophthalmol-2018-312910
Saidel MA, Paik JY, Garcia C et al (2012) Prevalence of sleep apnea syndrome and high-risk characteristics among keratoconus patients. Cornea 31(6):600–603. https://doi.org/10.1097/ICO.0b013e318243e446
Pihlblad MS, Schaefer DP (2013) Eyelid laxity, obesity, and obstructive sleep apnea in keratoconus. Cornea 32(9):1232–1236. https://doi.org/10.1097/ICO.0b013e318281e755
Gencer B, Ozgurhan EB, Kara S et al (2014) Obesity and obstructive sleep apnea in patients with keratoconus in a Turkish population. Cornea 33(2):137–140. https://doi.org/10.1097/ico.0000000000000024
Naderan M, Rezagholizadeh F, Zolfaghari M et al (2015) Association between the prevalence of obstructive sleep apnoea and the severity of keratoconus. Br J Ophthalmol 99(12):1675–1679. https://doi.org/10.1136/bjophthalmol-2015-306665
Woodward MA, Blachley TS, Stein JD (2016) The association between sociodemographic factors, common systemic diseases, and keratoconus: an analysis of a nationwide heath care claims database. Ophthalmology 123(3):457–465. https://doi.org/10.1016/j.ophtha.2015.10.035
Leveque TK, Yu L, Musch DC et al (2007) Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath 11(4):253–257. https://doi.org/10.1007/s11325-007-0112-3
Brodie FL, Charlson ES, Aleman TS et al (2015) (2015) Obstructive sleep apnea and central serous chorioretinopathy. Retina 35(2):238–243. https://doi.org/10.1097/IAE.0000000000000326
Chatziralli I, Kabanarou SA, Parikakis E et al (2017) Risk factors for central serous chorioretinopathy: multivariate approach in a case-control study. Curr Eye Res 42(7):1069–1073. https://doi.org/10.1080/02713683.2016.1276196
Liu PK, Chang YC, Tai MH et al (2020) The association between central serous chorioretinopathy and sleep apnea: a nationwide population-based study. Retina (Philadelphia, Pa.) 40(10):2034–2044. https://doi.org/10.1097/iae.0000000000002702
Chou KT, Huang CC, Tsai DC et al (2012) Sleep apnea and risk of retinal vein occlusion: a nationwide population-based study of Taiwanese. Am J Ophthalmol 154(1):200–205. https://doi.org/10.1016/j.ajo.2012.01.011e201
Agard E, El Chehab H, Vie AL et al (2018) Retinal vein occlusion and obstructive sleep apnea: a series of 114 patients. Acta Ophthalmol 96(8):e919–e925. https://doi.org/10.1111/aos.13798
Wang YH, Zhang P, Chen L et al (2019) Correlation between obstructive sleep apnea and central retinal vein occlusion. Int J Ophthalmol 12(10):1634–1636. https://doi.org/10.18240/ijo.2019.10.17
Wan W, Wu Z, Lu J et al (2017) Obstructive sleep apnea is related with the risk of retinal vein occlusion. Nat Sci Sleep 13:273–281. https://doi.org/10.2147/NSS.S290583
Onen SH, Mouriaux F, Berramdane L et al (2008) High prevalence of sleep-disordered breathing in patients with primary open-angle glaucoma. Acta OphthalmolScand 78(6):638–641
Marcus DM, Costarides AP, Gokhale P et al (2001) Sleep disorders: a risk factor for normal-tension glaucoma? J Glaucoma 10(3):177–183
Tsang CS, Chong SL, Ho CK et al (2006) Moderate to severe obstructive sleep apnoea patients is associated with a higher incidence of visual field defect. Eye (Lond) 20:38–42
Girkin CA, McGwin G Jr, McNeal SF et al (2006) Is there an association between pre-existing sleep apnoea and the development of glaucoma? Br J Ophthalmol 90(6):679–681. https://doi.org/10.1136/bjo.2005.086082
Sergi M, Salerno DE, Rizzi M, Blini M et al (2007) Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma 16(1):42–46. https://doi.org/10.1097/01.ijg.0000243472.51461.24
Boonyaleephan S, Neruntarat C (2009) The association of primary open-angle glaucoma/normal tension glaucoma and obstructive sleep apnea in Thai patients. J of Med and Health Sci 15(3):108–112
Roberts TV, Hodge C, Graham SL (2009) Prevalence of nocturnal oxygen desaturation and self-reported sleep-disordered breathing in glaucoma. J Glaucoma 18(2):114–118. https://doi.org/10.1097/IJG.0b013e318179f80c
Boyle-Walker M, Semes LP, Clay OJ et al (2011) Sleep apnea syndrome represents a risk for glaucoma in a veterans' affairs population. ISRN Ophthalmol 920767. https://doi.org/10.5402/2011/920767
Lin PW, Friedman M, Lin HC et al (2011) Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome. J Glaucoma 20(9):553–559. https://doi.org/10.1097/IJG.0b013e3181f3eb81
Nowak MS, Jurowski P, Gos R et al (2011) Pulsatile ocular blood flow in subjects with sleep apnoea syndrome. Arch Med Sci 7(2):332–336. https://doi.org/10.5114/aoms.2011.22087
Lin CC, Hu CC, Ho JD et al (2013) Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology 120(8):1559–1564. https://doi.org/10.1016/j.ophtha.2013.01.006
Moghimi S, Ahmadraji A, Sotoodeh H et al (2013) Retinal nerve fiber thickness is reduced in sleep apnea syndrome. Sleep Med 14(1):53–57. https://doi.org/10.1016/j.sleep.2012.07.004
Khandgave TP, Puthran N, Ingole AB et al (2013) The assessment of sleep apnoea as a risk factor in glaucoma. J Clin Diagn Res 7(7):1391–1393. https://doi.org/10.7860/JCDR/2013/5383.3147
Aptel F, Chiquet C, Tamisier R et al (2014) Sleep registry of the French Federation of Pneumology Paris F. Association between glaucoma and sleep apnea in a large French multicenter prospective cohort. Sleep Med 15(5):576–581. https://doi.org/10.1016/j.sleep.2013.11.790
Muniesa M, Sanchez-de-la-Torre M, Huerva V et al (2014) Floppy eyelid syndrome as an indicator of the presence of glaucoma in patients with obstructive sleep apnea. J Glaucoma 23(1):e81–e85. https://doi.org/10.1097/IJG.0b013e31829da19f
Bilgin G (2014) Normal-tension glaucoma and obstructive sleep apnea syndrome: a prospective study. BMC Ophthalmol 14:27. https://doi.org/10.1186/1471-2415-14-27
Bagabas N, Ghazali W, Mukhtar M et al (2019) Prevalence of glaucoma in patients with obstructive sleep apnea. J Epidemiol Glob Health 9(3):198–203. https://doi.org/10.2991/jegh.k.190816.001
Keenan TD, Goldacre R, Goldacre MJ (2019) Associations between obstructive sleep apnoea, primary open angle glaucoma and age-related macular degeneration: record linkage study. Br J Ophthalmol 101(2):155–159. https://doi.org/10.1136/bjophthalmol-2015-308278
Han X, Lee SSY, Ingold N et al (2021) Associations of sleep apnoea with glaucoma and age-related macular degeneration: an analysis in the United Kingdom Biobank and the Canadian longitudinal study on aging. BMC Med 19:104. https://doi.org/10.1186/s12916-021-01973-y
Fraser JA, Bruce BB, Rucker J et al (2009) Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci 290(1–2):86–89. https://doi.org/10.1016/j.jns.2009.11.001
Radojicic A, Vukovic-Cvetkovic V, Pekmezovic T et al (2019) Predictive role of presenting symptoms and clinical findings in idiopathic intracranial hypertension. J Neurol Sci 399:89–93. https://doi.org/10.1016/j.jns.2019.02.006
Ardissino M, Moussa O, Tang A et al (2019) Idiopathic intracranial hypertension in the British population with obesity. Acta Neurochir (Wien) 161(2):239–246. https://doi.org/10.1007/s00701-018-3772-9
Santos M, Hofmann RJ (2017) Ocular manifestations of obstructive sleep apnea. J Clin Sleep Med 13(11):1345–1348. https://doi.org/10.5664/jcsm.6812
Huon LK, Liu SYC, Camacho M et al (2016) The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 20:1145–1154. https://doi.org/10.1007/s11325-016-1358-4
Swartz MK (2011) The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care 25:1–2
Bulloch G, Seth I, Alphonse S et al (2022) Prevalence of obstructive sleep apnea with floppy eyelid syndrome: a systematic review and meta-analysis. Ophthalmic Plast Reconstr Surg. https://doi.org/10.1097/IOP.0000000000002298
Motamedi KK, McClary AC, Amedee RG (2009) Obstructive sleep apnea: a growing problem. Ochsner J 9(3):149–153
Archer EL, Pepin S (2013) Obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: evidence for an association. J Clin Sleep Med 9(6):613–618. https://doi.org/10.5664/jcsm.2766
De Gregorio A, Cerini A, Scala A et al (2021) Floppy eyelid, an under-diagnosed syndrome: a review of demographics, pathogenesis, and treatment. Ther Adv Ophthalmol 13:25158414211059250. https://doi.org/10.1177/25158414211059247
Pellegrini M, Bernabei F, Friehmann A et al (2020) Obstructive sleep apnea and keratoconus: a systematic review and meta-analysis. Optom Vis Sci 97(1):9–14. https://doi.org/10.1097/OPX.0000000000001467
Semeraro F, Morescalchi F, Russo A et al (2019) Central serous chorioretinopathy: pathogenesis and management. Clin Ophthalmol 13:2341–2352. https://doi.org/10.2147/OPTH.S220845
Wu CY, Riangwiwat T, Rattanawong P et al (2018) Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: a systematic review and meta-analysis. Retina 38(9):1642–1651. https://doi.org/10.1097/IAE.0000000000002117
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
This study was a systematic review and meta-analysis of already published literature. All included literature obtained institutional ethics and patient informed consent.
Human and animal rights
This study was a systematic review and meta-analysis of already published literature. All included literature complied with Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bulloch, G., Seth, I., Zhu, Z. et al. Ocular manifestations of obstructive sleep apnea: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol 262, 19–32 (2024). https://doi.org/10.1007/s00417-023-06103-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06103-3