Abstract
Background
Diquafosol enhances fluid transfer and mucin secretion on ocular surface, which has been suggested as an effective treatment for dry eye disease (DED). The aim of the systematic review and meta-analysis was to compare the efficacy and safety of topical diquafosol versus hyaluronic acid (HA) for DED.
Methods
Relevant randomized controlled trials were obtained via search of electronic including PubMed, Embase, Cochrane Library, and Web of Science. A random-effects model was used to pool the results after incorporating the influence of potential heterogeneity.
Results
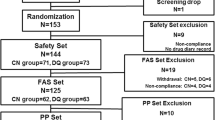
A total of nine RCTs involving 1295 patients with DED were included in the meta-analysis. Compared to treatment with 0.1% HA, topical treatment with 3% diquafosol significantly improved the Ocular Surface Disease Index (mean difference (MD): − 3.59, 95% confidence interval (CI): − 4.68 to − 2.50, p < 0.001; I2 = 6%), results of Schirmer’s test (MD: 1.08 mm, 95% CI: 0.41 to 1.76, p = 0.002; I2 = 0%), tear breakup time (MD: 0.60 s, 95% CI: 0.20 to 0.99, p = 0.003; I2 = 63%), corneal fluorescein staining score (MD: − 0.20, 95% CI: − 0.37 to − 0.03, p = 0.02; I2 = 58%), and ocular rose bengal staining score (MD: − 0.62, 95% CI: − 0.88 to − 0.35, p < 0.001; I2 = 15%). No severe adverse events were reported. Topical use of diquafosol was associated with a higher risk of overall adverse events as compared to HA (odds ratio: 1.71, 95% CI: 1.08 to 2.71, p = 0.02; I2 = 18%).
Conclusions
Topical treatment with 3% diquafosol may be more effective than 0.1% HA for patients with DED. However, the long-term efficacy and tolerability of diquafosol still need to be determined.





Similar content being viewed by others
References
Cai Y, Wei J, Zhou J, Zou W (2022) Prevalence and incidence of dry eye disease in Asia: a systematic review and meta-analysis. Ophthalmic Res 65:647–658. https://doi.org/10.1159/000525696000525696
Qian L, Wei W (2022) Identified risk factors for dry eye syndrome: a systematic review and meta-analysis. PloS One 17:e0271267. https://doi.org/10.1371/journal.pone.0271267
Fjaervoll K, Fjaervoll H, Magno M, Noland ST, Dartt DA, Vehof J, Utheim TP (2022) Review on the possible pathophysiological mechanisms underlying visual display terminal-associated dry eye disease. Acta Ophthalmol 100:861–877. https://doi.org/10.1111/aos.15150AOS15150
Sekhon AS, He B, Iovieno A, Yeung SN (2021) Pathophysiology of corneal endothelial cell loss in dry eye disease and other inflammatory ocular disorders. Ocul Immunol Inflamm 1-11. https://doi.org/10.1080/09273948.2021.1980808
Basilious A, Xu CY, Malvankar-Mehta MS (2022) Dry eye disease and psychiatric disorders: a systematic review and meta-analysis. Eur J Ophthalmol 32:1872–1889. https://doi.org/10.1177/11206721211060963
Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK, Varu DM, Musch DC, Dunn SP, Mah FS (2019) American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Ophthalmology 126:P286–P334. https://doi.org/10.1016/j.ophtha.2018.10.023
Rolando M, Merayo-Lloves J (2022) Management strategies for evaporative dry eye disease and future perspective. Curr Eye Res 47:813–823. https://doi.org/10.1080/02713683.2022.2039205
Sheppard J, Shen Lee B, Periman LM (2023) Dry eye disease: identification and therapeutic strategies for primary care clinicians and clinical specialists. Ann Med 55:241–252. https://doi.org/10.1080/07853890.2022.2157477
Yang YJ, Lee WY, Kim YJ, Hong YP (2021) A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int J Environ Res Public Health 18:2383. https://doi.org/10.3390/ijerph18052383
Hynnekleiv L, Magno M, Vernhardsdottir RR, Moschowits E, Tonseth KA, Dartt DA, Vehof J, Utheim TP (2022) Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol 100:844–860. https://doi.org/10.1111/aos.15159AOS15159
Keating GM (2015) Diquafosol ophthalmic solution 3 %: a review of its use in dry eye. Drugs 75:911–922. https://doi.org/10.1007/s40265-015-0409-7
Nakamura M, Imanaka T, Sakamoto A (2012) Diquafosol ophthalmic solution for dry eye treatment. Adv Ther 29:579–589. https://doi.org/10.1007/s12325-012-0033-9
Kamiya K, Nakanishi M, Ishii R, Kobashi H, Igarashi A, Sato N, Shimizu K (2012) Clinical evaluation of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter study. Eye (Lond) 26:1363–1368. https://doi.org/10.1038/eye.2012.166eye2012166
Takamura E, Tsubota K, Watanabe H, Ohashi Y (2012) A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol 96:1310–1315. https://doi.org/10.1136/bjophthalmol-2011-301448
Hwang HS, Sung YM, Lee WS, Kim EC (2014) Additive effect of preservative-free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea 33:935–941. https://doi.org/10.1097/ICO.0000000000000213
Toda I, Ide T, Fukumoto T, Ichihashi Y, Tsubota K (2014) Combination therapy with diquafosol tetrasodium and sodium hyaluronate in patients with dry eye after laser in situ keratomileusis. Am J Ophthalmol 157(616-622):e611. https://doi.org/10.1016/j.ajo.2013.11.017
Gong L, Sun X, Ma Z, Wang Q, Xu X, Chen X, Shao Y, Yao K, Tang L, Gu Y, Yuan H, Chua WH, Chuan JC, Tong L (2015) A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br J Ophthalmol 99:903–908. https://doi.org/10.1136/bjophthalmol-2014-306084
Park DH, Chung JK, Seo DR, Lee SJ (2016) Clinical effects and safety of 3% diquafosol ophthalmic solution for patients with dry eye after cataract surgery: a randomized controlled trial. Am J Ophthalmol 163(122-131):e122. https://doi.org/10.1016/j.ajo.2015.12.002
Cui L, Li Y, Lee HS, Yang JM, Choi W, Yoon KC (2018) Effect of diquafosol tetrasodium 3% on the conjunctival surface and clinical findings after cataract surgery in patients with dry eye. Int Ophthalmol 38:2021–2030. https://doi.org/10.1007/s10792-017-0693-1
Jun I, Choi S, Lee GY, Choi YJ, Lee HK, Kim EK, Seo KY, Kim TI (2019) Effects of preservative-free 3% diquafosol in patients with pre-existing dry eye disease after cataract surgery: a randomized clinical trial. Sci Rep 9:12659. https://doi.org/10.1038/s41598-019-49159-01265910
Zhang Q, Zhang H, Qin G, Wu Y, Song Y, Yang L, Yu S, He X, Moore JE, Moutari S, Palme C, Xu L, He W, Pazo EE (2022) Impact of diquafosol ophthalmic solution on tear film and dry eye symptom in type 2 diabetic dry eye: a pilot study. J Ocul Pharmacol Ther 38:133–140. https://doi.org/10.1089/jop.2021.0083
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. https://doi.org/10.1136/bmj.n160
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. www.training.cochrane.org/handbook
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Wu D, Chen WQ, Li R, Wang Y (2015) Efficacy and safety of topical diquafosol ophthalmic solution for treatment of dry eye: a systematic review of randomized clinical trials. Cornea 34:644–650. https://doi.org/10.1097/ICO.0000000000000429
Zhao X, Xia S, Chen Y (2017) Comparison of the efficacy between topical diquafosol and artificial tears in the treatment of dry eye following cataract surgery: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 96:e8174. https://doi.org/10.1097/md.0000000000008174
Nam K, Kim HJ, Yoo A (2019) Efficacy and safety of topical 3% diquafosol ophthalmic solution for the treatment of multifactorial dry eye disease: meta-analysis of randomized clinical trials. Ophthalmic Res 61:188–198. https://doi.org/10.1159/000492896000492896[pii]
Kojima T, Dogru M, Ibrahim OM, Nagata T, Higa K, Shimizu T, Shirasawa T, Satake Y, Shimazaki S, Shimazaki J, Tsubota K (2014) The effects of 3% diquafosol sodium application on the tear functions and ocular surface of the Cu,Zn-superoxide dismutase-1 (Sod1)-knockout mice. Mol Vis 20:929–938
Byun YS, Yoo YS, Kwon JY, Joo JS, Lim SA, Whang WJ, Mok JW, Choi JS, Joo CK (2016) Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp Eye Res 143:89–97. https://doi.org/10.1016/j.exer.2015.10.013
Ikeda K, Simsek C, Kojima T, Higa K, Kawashima M, Dogru M, Shimizu T, Tsubota K, Shimazaki J (2018) The effects of 3% diquafosol sodium eye drop application on meibomian gland and ocular surface alterations in the Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. Graefes Arch Clin Exp Ophthalmol 256:739–750. https://doi.org/10.1007/s00417-018-3932-x10
Park JH, Moon SH, Kang DH, Um HJ, Kang SS, Kim JY, Tchah H (2018) Diquafosol sodium inhibits apoptosis and inflammation of corneal epithelial cells via activation of Erk1/2 and RSK: in vitro and in vivo dry eye model. Invest Ophthalmol Vis Sci 59:5108–5115. https://doi.org/10.1167/iovs.17-229252711318
Endo KI, Sakamoto A, Fujisawa K (2021) Diquafosol tetrasodium elicits total cholesterol release from rabbit meibomian gland cells via P2Y(2) purinergic receptor signalling. Sci Rep 11:6989. https://doi.org/10.1038/s41598-021-86433-66989
Katagiri A, Tsubota K, Mikuzuki L, Nakamura S, Toyofuku A, Kato T, Bereiter DA, Iwata K (2023) Diquafosol sodium reduces neuronal activity in trigeminal subnucleus caudalis in a rat model of chronic dry eye disease. Neurosci Lett 792:136939. https://doi.org/10.1016/j.neulet.2022.136939
Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K (2012) Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology 119:1954–1960. https://doi.org/10.1016/j.ophtha.2012.04.010
Gross D, Childs M, Piaton JM (2017) Comparison of 0.2% and 0.18% hyaluronate eye drops in patients with moderate to severe dry eye with keratitis or keratoconjunctivitis. Clin Ophthalmol 11:631–638. https://doi.org/10.2147/OPTH.S131384opth-11-631
Park Y, Song JS, Choi CY, Yoon KC, Lee HK, Kim HS (2017) A randomized multicenter study comparing 0.1%, 0.15%, and 0.3% sodium hyaluronate with 0.05% cyclosporine in the treatment of dry eye. J Ocul Pharmacol Ther 33:66–72. https://doi.org/10.1089/jop.2016.0086
Aragona P, Simmons PA, Wang H, Wang T (2019) Physicochemical properties of hyaluronic acid-based lubricant eye drops. Transl Vis Sci Technol 8:2. https://doi.org/10.1167/tvst.8.6.22TVST-19-1425
Berg EJ, Ying GS, Maguire MG, Sheffield PE, Szczotka-Flynn LB, Asbell PA, Shen JF (2020) Climatic and environmental correlates of dry eye disease severity: a report from the Dry Eye Assessment and Management (DREAM) Study. Transl Vis Sci Technol 9:25. https://doi.org/10.1167/tvst.9.5.2525TVST-19-1699
Author information
Authors and Affiliations
Contributions
Xiaonan Sun and Chi Liu designed the study. Xiaonan Sun and Lei Liu performed the literature search, study selection, quality evaluation, and data extraction. Xiaonan Sun and Chi Liu performed the statistical analyses and interpreted the results. Xiaonan Sun drafted the manuscript. All authors revised the manuscript and approved the submission.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Liu, L. & Liu, C. Topical diquafosol versus hyaluronic acid for the treatment of dry eye disease: a meta-analysis of randomized controlled trials. Graefes Arch Clin Exp Ophthalmol 261, 3355–3367 (2023). https://doi.org/10.1007/s00417-023-06083-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06083-4