Abstract
Purpose
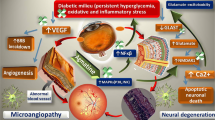
Angiogenesis in diabetic retinopathy (DR) is associated with increased retinal expression of angiopoietin-2 (Ang-2) and protein kinase C (PKC). Tocotrienol-rich fraction (TRF) has been shown to reduce the expression vascular endothelial growth factor (VEGF) in several experimental models. However, its effect against other angiogenic markers such as Ang-2 and PKC in rat model of diabetes remains unknown. Therefore, we investigated the effect of TRF on the retinal vascular changes and Ang-2 and PKC expressions in rats with streptozotocin (STZ)-induced DR.
Methods
Sprague–Dawley rats were divided into normal control rats (N) which received vehicle, and diabetic rats which either received vehicle (DV) or 100 mg/kg of TRF (DT). Diabetes was induced with intraperitoneal injection of STZ (60 mg/kg body weight). Treatments were given orally, once daily, for 12 weeks after confirmation of hyperglycaemia. Fundus photographs were captured at baseline, 6- and 12-week post-STZ injection and average diameter of retinal veins and arteries were measured. At 12-week post-STZ injection, rats were euthanised, and retinae were collected for measurement of Ang-2 and PKC gene and protein expressions.
Results
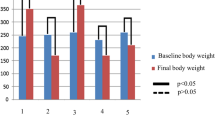
Retinal venous and arterial diameters were significantly greater in DV compared to DT at week 12 post-STZ injection (p < 0.001 and < 0.05, respectively). The vessel diameter measurements in DT were comparable to N and this effect of TRF was associated with significantly lower Ang-2 and PKC gene and protein expressions compared to DV.
Conclusion
Oral TRF reduces the expression of retinal angiogenic markers and preserves the retinal vascular diameter of rats with STZ-induced DR.





Similar content being viewed by others
Data transparency
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris III FL, Klein R, American Diabetes Association (2004) Retinopathy in diabetes. Diabetes Care 27(suppl_1):s84-s87.
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1984) The Wisconsin Epidemiologic Study of Diabetic Retinopathy: III Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 102(4):527–532
Klein R, Klein BE, Moss SE (1984) Visual impairment in diabetes. Ophthalmology 91(1):1–9
Peters S, Cree IA, Alexander R, Turowski P, Ockrim Z, Patel J, Boyd SR, Joussen AM, Ziemssen F, Hykin PG, Moss SE (2007) Angiopoietin modulation of vascular endothelial growth factor: effects on retinal endothelial cell permeability. Cytokine 40(2):144–150
Jin SW, Patterson C (2009) The opening act: vasculogenesis and the origins of circulation. Arterioscler Thromb Vasc Biol 29(5):623–629
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87(7):1171–1180
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87(7):1161–1169
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277(5322):55–60
Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H (2005) Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 139(3):476–481
Bossenmaier B, Mosthaf L, Mischak H, Ullrich A, Häring HU (1997) Protein kinase C isoforms β 1 and β 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia 40(7):863–866
Williams B, Gallacher B, Patel H, Orme C (1997) Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 46(9):1497–1503
Shibata A, Nakagawa K, Tsuduki T, Miyazawa T (2015) δ-Tocotrienol treatment is more effective against hypoxic tumor cells than normoxic cells: potential implications for cancer therapy. J Nutr Biochem 26(8):832–840
Krager KJ, Pineda EN, Kharade SV, Kordsmeier M, Howard L, Breen PJ, Compadre CM, Hauer-Jensen M, Aykin-Burns N (2015) Tocotrienol-rich fraction from rice bran demonstrates potent radiation protection activity. Evid Based Complement Alternat Med 2015:ID148791.
Minhajuddin M, Beg ZH, Iqbal J (2005) Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem Toxicol 43(5):747–753
Sundram K, Sambanthamurthi R, Tan YA (2003) Palm fruit chemistry and nutrition. Asia Pac J Clin Nutr 12(3):355–362
Heinonen M, Piironen V (1991) The tocopherol, tocotrienol, and vitamin E content of the average Finnish diet. Int J Vitam Nutr Res 61(1):27–32
Sailo BL, Banik K, Padmavathi G, Javadi M, Bordoloi D, Kunnumakkara AB (2018) Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol Res 130:259–272
Thomsen CB, Andersen RF, Steffensen KD, Adimi P, Jakobsen A (2019) Delta tocotrienol in recurrent ovarian cancer. A phase II trial Pharmacol Res 141:392–396
Sadikan MZ, Abdul Nasir NA, Agarwal R, Mohd Ismail N (2020) Protective effect of palm oil-derived tocotrienol-rich fraction against retinal neurodegenerative changes in rats with streptozotocin-induced diabetic retinopathy. Biomolecules 10(4):556
Furman BL (2021) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc 1(4):e78
Abdul Nasir NA, Agarwal R, Sheikh Abdul Kadir SH, Vasudevan S, Tripathy M, Iezhitsa I, Mohammad Daher A, Ibrahim MI, Mohd Ismail N (2017) Reduction of oxidative-nitrosative stress underlies anticataract effect of topically applied tocotrienol in streptozotocin-induced diabetic rats. PLoS ONE 12(3):e0174542
Meighan SS (1956) Blood vessels of the bulbar conjunctiva. Br J Ophthalmol 40(9):513–526
Cohan BE, Pearch AC, Jokelainen PT, Bohr DF (2003) Optic disc imaging in conscious rats and mice. Invest Opthalmol Vis Sci 44(1):160–163
Takai Y, Tanito M, Omura T, Kawasaki R, Kawasaki Y, Ohira A (2017) Comparisons of retinal vessel diameter and glaucomatous parameters between both eyes of subjects with clinically unilateral pseudoexfoliation syndrome. PLoS ONE 12(6):e0179663
Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J (2009) The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics 64(3):235–244
Jakus V, Bauerova K, Michalkova D, Carsky J (2000) Values of markers of early and advanced glycation and lipoxidation in serum proteins of children with diabetes mellitus. Bratisl Lek Listy 101(9):484–489
Osaadon P, Fagan XJ, Lifshitz T, Levy J (2014) A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 28(5):510–520
Diabetic Retinopathy Study Research Group (1981) Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 88(7):583–600
Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, Palta M (2003) Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology 110(11):2118–2125
Roy MS, Klein R, Janal MN (2011) Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with type 1 diabetes mellitus. Arch Ophthalmol 129(1):8–15
Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR (2001) Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet 358(9288):1134–1140
Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD (2002) Retinal arteriolar narrowing and risk of coronary heart disease in men and women: the Atherosclerosis Risk in Communities Study. JAMA 287(9):1153–1159
Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S (2011) Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther 27(2):123–130
Quinn R (2005) Comparing rat’s to human’s age: how old is my rat in people years? Nutrition 21(6):775–777
Patel V, Rassam S, Newsom R, Wiek J, Kohner E (1992) Retinal blood flow in diabetic retinopathy. BMJ-Brit Med J 305(6855):678–683
Drobnjak D, Munch IC, Glümer C, Færch K, Kessel L, Larsen M, Veiby NC (2017) Relationship between retinal vessel diameters and retinopathy in the Inter99 Eye Study. J Clin Transl Endocrinol 8:22–28
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27(4):331–371
Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S, Yancopoulos G, Zack DJ, Campochiaro PA (2004) Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood–retinal barrier. Gene Ther 11(10):865–873
Khalaf N, Helmy H, Fahmy I, Abd El Hamid M, Moemen L (2017) Role of angiopoietins and Tie-2 in diabetic retinopathy. Electron physician 9(8):5031–5035
Mandriota SJ, Pepper MS (1998) Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 83(8):852–859
Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y (1994) Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 274(22):15732–15739
Holash JM, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284(5422):1994–1998
Pfister F, Wang Y, Schreiter K, Vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y (2010) Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol 47(1):59–64
Lee SG, Lee CG, Yun IH, Hur DY, Yang JW, Kim HW (2012) Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin Experiment Ophthalmol 40(1):e47–e57
Jiang Q (2014) Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 72:76–90
Strain WD, Chaturvedi N (2002) The renin-angiotensin-aldosterone system and the eye in diabetes. J Renin Angiotensin Aldosterone Syst 3(4):243–246
Drury PL, Bodansky HJ, Oddie CJ, Cudworth AG, Edwards CR (1982) Increased plasma renin activity in type 1 diabetes with microvascular disease. Clin Endocrinol 16(5):453–461
Murata M, Nakagawa M, Takahashi S (1997) Expression and localization of angiotensin II type 1 receptor mRNA in rat ocular tissues. Ophthalmologica 211(6):384–386
Otani A, Takagi H, Oh H, Koyama S, Honda Y (2001) Angiotensin II induces expression of the Tie2 receptor ligand, angiopoietin-2, in bovine retinal endothelial cells. Diabetes 50(4):867–875
Nishizuka Y (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258(5082):607–614
Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9(7):484–496
Acknowledgements
We acknowledge the administrative and facility support by Research Management Centre, Institute of Medical Molecular Biotechnology (IMMB) and Laboratory Animal Care Unit (LACU), Universiti Teknologi MARA, Malaysia.
Funding
This work was supported by grant from Fundamental Research Grant Scheme by Ministry of Higher Education (MoHE) (600-IRMI/FRGS 5/3 (101/2019)) and Malaysian Society of Ophthalmology small research grant.
Author information
Authors and Affiliations
Contributions
NAAG, NAAN, and RA conceived the research and designed the experiment. NAAG, MZS, and LL performed experiments and analysis. NAAN, RA, and NR participated in the interpretation of the data. NAAG drafted the manuscript. NAAN, RA, and NR supervised and revised the manuscript. All authors contributed to the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the local institutional animal ethics committee (Universiti Teknologi MARA Committee of Animal Research and Ethics) under approval number 3/2019/(286/2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors have jointly decided to designate NAAN to be responsible for decision-making regarding the presence of authors and the order of their presence in the manuscript. NAAN has also been selected by all authors to be responsible for all future communication with the journal regarding this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdul Ghani, N.A., Abdul Nasir, N.A., Lambuk, L. et al. The effect of palm oil-derived tocotrienol-rich fraction in preserving normal retinal vascular diameter in streptozotocin-induced diabetic rats. Graefes Arch Clin Exp Ophthalmol 261, 1587–1596 (2023). https://doi.org/10.1007/s00417-022-05965-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05965-3