Abstract
Purpose
Assess correlation between change in central subfield thickness (CST) and change in best-corrected visual acuity (BCVA) in eyes with macular edema due to retinal vein occlusion (RVO) that received intravitreal aflibercept injections (IAI).
Methods
Post hoc analysis of COPERNICUS and GALILEO trials for CRVO and VIBRANT trial for BRVO with relationships determined using Pearson correlation coefficient.
Results
In COPERNICUS, correlations (r) between change in CST and change in BCVA from baseline at weeks 12, 24, 52, and 100 were −0.36 (95% CI: −0.52, −0.18; P < 0.001), −0.38 (95% CI: −0.53, −0.20; P < 0.001), −0.44 (95% CI: −0.58, −0.27; P < 0.001), and −0.41 (95% CI: −0.56, −0.23; P < 0.001), respectively. CST changes accounted for only 21% of the variance in BCVA changes; every 100-µm decrease in CST was associated with a 2.1-letter increase in BCVA (P = 0.003). Similar findings were noted for GALILEO (r, −0.45 to −0.23) and VIBRANT (r, −0.36 to −0.32) trials.
Conclusion
In eyes treated with IAI for macular edema due to RVO, correlation between change in CST and change in BCVA was weak to moderate. While change in CST may be helpful in determining the need for anti-VEGF therapy, these findings do not support using changes in CST as a surrogate for changes in visual acuity outcomes.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Objective parameters, such as central subfield thickness (CST) noted on optical coherence tomography (OCT), have been suggested as biomarkers for predicting visual outcomes in retinal diseases. In eyes with retinal vein occlusion (RVO) with macular edema treated with anti-vascular endothelial growth factor (anti-VEGF) agents, there have been conflicting conclusions regarding whether CST correlates with visual acuity (VA), potentially related to limitations or interpretations of methods used. Previous studies have also shown mixed results regarding the prognostic value of baseline CST for visual outcomes [1,2,3,4,5]. Fewer studies have evaluated the correlation between change in CST and change in VA, and findings have been similarly mixed. In a prospective study of eyes with RVO treated with bevacizumab, Hoeh et al. showed a moderate correlation between change in central retinal thickness (CRT) and change in VA in branch RVO (BRVO), but none in central RVO (CRVO) [2]. Spaide et al. also observed no correlation between change in CRT and change in VA in a prospective case series of eyes with CRVO treated with ranibizumab [6]. In contrast, in a retrospective analysis of clinical trial data of eyes with RVO treated with ranibizumab, Ou et al. showed a moderate correlation between change in CRT and change in VA in CRVO, but none in BRVO [7]. Each of these studies had several limitations, including the following: (1) small numbers of participants which could limit the ability to identify correlations, (2) little, if any, standardization of refraction or visual acuity measurements, (3) little standardization or adherence to visit or treatment regimens, and (4) no data using fixed dosing regimens.
The correlation between change in CST and change in VA in eyes with macular edema due to RVO treated with anti-VEGF agents has not been examined extensively, to our knowledge, in large randomized clinical trials and may provide guidance for management. This investigation aimed to assess this correlation in a post hoc analysis of eyes with macular edema due to RVO treated with intravitreal aflibercept injections (IAI) from three randomized clinical trials. These included the COPERNICUS and GALILEO clinical trials, in which eyes with macular edema due to CRVO received IAI over 100 weeks (COPERNICUS) or 76 weeks (GALILEO), as well as the VIBRANT clinical trial, in which eyes with macular edema due to BRVO received IAI over 52 weeks.
Methods
Study designs of COPERNICUS, GALILEO, and VIBRANT
All three RVO studies were phase 3, randomized, double-masked, multicenter clinical trials. COPERNICUS (ClinicalTrials.gov identifier no. NCT00943072) was conducted across 61 sites in the USA, Canada, Colombia, India, and Israel. GALILEO (ClinicalTrials.gov identifier no. NCT01012973) was carried out at 63 sites across Europe and Asia/Pacific. VIBRANT (ClinicalTrials.gov identifier no. NCT01521559) was conducted at 58 sites in North America and Japan [8,9,10]. All respective institutional review boards/ethics committees approved the study protocols, which were carried out in compliance with ethical guidelines of the Declaration of Helsinki, the International Conference on Harmonization guidelines for Good Clinical Practice, and, for US patients, the Health Insurance Portability and Accountability Act of 1996. All participants provided written informed consent before the initiation of the study-specific procedures [8,9,10]. This post hoc analysis was designed starting October 1, 2020, and completed on January 31, 2021, with IRB agreement from the Johns Hopkins University School of Medicine that the human subject research was exempt as it only used de-identified data from clinical trial participants whose written informed consent document, captured during the original informed consent process, included permission for using de-identified information for post hoc analyses like this investigation.
The design and eligibility criteria for all three trials have been reported previously. [8,9,10] Only one eye from each patient was included in these studies. COPERNICUS and GALILEO were 100-week and 76-week studies, respectively, that randomized patients with macular edema secondary to CRVO in a 3:2 ratio to receive IAI 2 mg (IAI 2q4) or sham injections every 4 weeks through week 24, for a total of six doses. [8, 9]
In COPERNICUS, from weeks 24 to 52, all study participants were evaluated monthly and received IAI pro re nata (PRN) based on prespecified re-treatment criteria [8]. From weeks 52 to 100, study participants from both study arms were evaluated at least quarterly and received IAI PRN according to the same re-treatment criteria. Participants could be evaluated and dosed as frequently as every 4 weeks if deemed necessary by the investigators.
In GALILEO, from weeks 24 to 48, patients in the IAI 2q4 group were evaluated every 4 weeks and received IAI PRN if they met similar prespecified re-treatment criteria as in COPERNICUS. Participants in the sham group continued to receive sham at all scheduled visits through week 48. From weeks 52 to 76, patients in both intravitreal aflibercept (IAI 2q4/IAI PRN) and sham (sham/IAI PRN) groups were monitored every 8 weeks and received intravitreal aflibercept PRN according to the same prespecified re-treatment criteria. [9]
VIBRANT was a 52-week trial that enrolled patients with BRVO or hemi-retinal vein occlusion with foveal center-involved macular edema [10]. Study participants were randomized 1:1 to receive either laser at baseline or IAI 2q4 from baseline through week 24 followed by IAI every 8 weeks (2q8) through week 52. Starting from week 12, patients in both treatment groups could receive rescue treatment based on prespecified criteria [10]. Eyes in the IAI group eligible for rescue treatment received active laser at week 36. Eyes in the laser group, if eligible for rescue treatment before week 24, received one additional laser from week 12 to week 20 and, if eligible for rescue treatment after week 24, received IAI 2q8 after three initial monthly doses.
Post hoc analysis
This post hoc analysis evaluated the association between CST and BCVA as well as the association between changes in CST and changes in BCVA from baseline in participants with macular edema secondary to RVO treated with sham, laser, or IAI. All participants who were in the full analysis set (defined as patients who were randomized and had baseline BCVA and at least one post-baseline BCVA assessment) and had BCVA and CST measurements at baseline were included in this analysis. CST was measured by time domain (TD)-OCT in study participants with CRVO in COPERNICUS and GALILEO studies and by spectral domain (SD)-OCT in participants with BRVO in VIBRANT.
Statistical analyses
Data from COPERNICUS, GALILEO, and VIBRANT studies were analyzed separately. An initial analysis of data indicated that both absolute and changes in BCVA and CST showed a normal distribution across all treatment groups. Hence, Pearson correlation coefficient was used to evaluate the association between CST and BCVA at baseline and weeks 12, 24, 52, and 100 as well as between changes in CST and changes in BCVA at weeks 12, 24, 52, and 100 for COPERNICUS. Similar analyses were performed for GALILEO (at baseline and weeks 12, 24, 52, and 76) and VIBRANT (at baseline and weeks 12, 24, and 52). The 95% confidence intervals (CIs) of the Pearson correlation coefficients (r) were estimated using Fisher z transformation, and the relationship between the two variables was considered negligible for (r) from 0 to < 0.1, weak for (r) from 0.1 to < 0.40, moderate for (r) from 0.4 to < 0.7, strong for (r) from 0.7 to < 0.9, and very strong for (r) from 0.9 to 1 [11]. Generalized linear regression models were used to calculate the slope of the regression line for change in CST and the coefficient of determination (R2) for each visit. For all three trials, at the final study visit, previously identified baseline factors (age, BCVA, perfusion status, and time since diagnosis) that may be associated with BCVA changes and may be confounding variables were included in the models. Observed data were used at each time point. Data were censored after rescue medication was given. All P values reported were 2-sided, and no adjustments were made for multiplicity. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
COPERNICUS
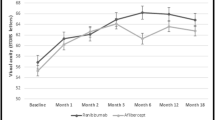
Of 187 eyes in the full analysis set (FAS), 114 eyes were randomized to receive IAI from the start of the trial. The percentage of eyes with both BCVA and CST measurements available for analysis at weeks 12, 24, 52, and 100 was 94%, 92%, 92%, and 88%. At baseline, the correlation (r) between CST and BCVA was −0.50 (95% CI: −0.63 to −0.34; P < 0.001). At weeks 12, 24, 52, and 100, the r values were −0.10 (95% CI: −0.28 to 0.10; P = 0.33), 0.19 (95% CI: −0.00 to 0.37; P = 0.05), −0.24 (95% CI: −0.41 to −0.05; P = 0.01), and −0.24 (95% CI: −0.42 to −0.05; P = 0.01), respectively (Table 1). Supplementary Figs. 1 and 2 show the linear regression between CST and VA at baseline and weeks 12, 24, 52, and 100 for the sham and IAI groups.
Correlations (r) between change in CST and change in BCVA from baseline at weeks 12, 24, 52, and 100 were −0.36 (95% CI: −0.52 to −0.18; P < 0.001), −0.38 (95% CI: −0.53 to −0.20; P < 0.001), −0.44 (95% CI: −0.58 to −0.27; P < 0.001), and −0.41 (95% CI: −0.56 to −0.23; P < 0.001), respectively (Table 1 and Fig. 1; see Supplementary Fig. 1, for the sham group). In a linear regression analysis of correlation between change in CST and change in BCVA at week 100 adjusted for baseline factors (age, perfusion status, time since diagnosis, and baseline BCVA), CST changes accounted for only 21% of the variance in BCVA changes. At 100 weeks, every 100-µm decrease in CST was associated with a 2.1-letter increase in BCVA; of note, however, the confidence interval was large (95% CI: 0.8 to 3.5; P = 0.003; Supplementary Table 1).
Correlations between changes in CST and changes in BCVA from baseline in the IAI group in the COPERNICUS trial. Solid lines indicate the correlation line, and dashed lines indicate the 95% CIs. In COPERNICUS, patients with macular edema secondary to CRVO received IAI 2q4 or sham injections every 4 weeks through week 24, for a total of six doses. From weeks 24 to 100, all study patients received IAI pro re nata (PRN) based on prespecified re-treatment criteria. 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks; BCVA, best-corrected visual acuity; CI, confidence interval; CST, central subfield thickness; IAI, intravitreal aflibercept injection; PRN, pro re nata; r, correlation; R.2, coefficient of determination
GALILEO
Of 171 eyes in the FAS, 103 eyes were randomized to receive IAI from the start of the trial. The percentage of eyes with both BCVA and CST measurements available for analysis at weeks 12, 24, 52, and 76 was 93%, 94%, 86%, and 84%. At baseline, the correlation (r) between CST and BCVA was −0.23 (95% CI: −0.40, −0.04; P = 0.02). At weeks 12, 24, 52, and 76, the r values were −0.07 (95% CI: −0.26, 0.14; P = 0.52), −0.04 (95% CI: −0.24, 0.16; P = 0.70), −0.13 (95% CI: −0.33, 0.09; P = 0.24), and −0.52 (95% CI: −0.66, −0.35; P < 0.001), respectively (Supplementary Table 2).
Correlations (r) between change in CST and change in BCVA from baseline at weeks 12, 24, 52, and 76 for the IAI group were −0.30 (95% CI: −0.47 to −0.11; P = 0.003), −0.23 (95% CI: −0.41 to −0.03; P = 0.02), −0.40 (95% CI: −0.56 to −0.20; P = 0.001), and −0.45 (95% CI: −0.60 to −0.26; P < 0.001), respectively (Supplementary Table 2 and Figs. 4 through 7). In a linear regression analysis of correlation between change in CST and change in BCVA at week 76 adjusted for baseline factors (age, perfusion status, time since diagnosis, and baseline BCVA), CST changes accounted for only 33% of the variance in BCVA changes. At 76 weeks, every 100-µm decrease in CST was associated with a 2.4-letter increase in BCVA; of note, however, the confidence interval was large (95% CI: 1.3 to 3.5, P < 0.001; Supplementary Table 1).
VIBRANT
Of 181 eyes in the FAS, 91 eyes were randomized to receive IAI from the start of the trial. The percentage of eyes with both BCVA and CST measurements available for analysis at weeks 12, 24, and 52 was 97%, 91%, and 80%. At baseline, the correlation (r) between CST and BCVA was −0.41 (95% CI: −0.56 to −0.22; P < 0.001). At weeks 12, 24, and 52, the r values were 0.13 (95% CI: −0.08 to 0.33; P = 0.22), 0.14 (95% CI: −0.08 to 0.35; P = 0.20), and 0.17 (95% CI: −0.06 to 0.39; P = 0.14), respectively (Table 2). Supplementary Figs. 8 and 9 show the linear regression between CST and VA at baseline and weeks 12, 24, and 52 for the laser/IAI and IAI groups.
Correlations (r) between change in CST and change in BCVA from baseline at weeks 12, 24, and 52 were −0.34 (95% CI: −0.51 to −0.14; P = 0.001), −0.32 (95% CI: −0.50 to −0.11; P = 0.003), and −0.36 (95% CI: −0.54 to −0.14; P = 0.002), respectively (Table 2 and Fig. 2; see Supplementary Fig. 10). In a linear regression analysis of correlation between change in CST and change in BCVA at week 52 adjusted for baseline factors (age, perfusion status, time since diagnosis, and baseline BCVA), CST changes accounted for only 23% of the variance in BCVA changes. At 52 weeks, every 100-µm decrease in CST was associated with a 2.2-letter increase in BCVA; of note, however, the confidence interval was large (95% CI: −0.2 to 4.5; P = 0.07; Supplementary Table 1).
Correlations between changes in CST and changes in BCVA from baseline in the IAI group in the VIBRANT trial. Solid lines indicate the correlation line, and dashed lines indicate the 95% CIs. In VIBRANT, patients with macular edema secondary to BRVO received either laser at baseline or IAI 2q4 from baseline through week 24. Both treatment groups received IAI 2q8 from week 24 through week 52. 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks; BCVA, best-corrected visual acuity; CI, confidence interval; CST, central subfield thickness; IAI, intravitreal aflibercept injection; r, correlation; R.2, coefficient of determination
Discussion
The findings of this study suggest that the association between CST and visual outcomes at baseline in eyes with macular edema due to RVO is weak to moderate for CRVO (r, −0.50 to −0.23) and moderate for BRVO (r, −0.41). These findings are comparable to those shown in the Standard Care Versus Corticosteroid for Retinal Vein Occlusion (SCORE) study, which reported a correlation coefficient of −0.27 for CRVO and −0.28 for BRVO [12]. In contrast, Ou et al. reported no correlation between CRT and VA at baseline in the Rubeosis Anti-VEGF (RAVE) and the Wide-field Angiography Guided Targeted Retinal Photocoagulation Combined with Anti-VEGF Intravitreal Injections for the Treatment of Ischemic Retinal Vein Occlusion (WAVE) trials [7].
Regarding the correlation between change in CST and change in VA from baseline at follow-up using a fixed-dosing regimen, the present study found a weak to moderate correlation (r, 0.45 to −0.23) for CRVO. Ou et al. reported a correlation coefficient of −0.43 at 12 months; similarly, the present study found correlation coefficients of −0.40 to −0.44 at week 52. By comparison, other studies have not been able to demonstrate a correlation between change in CST and change in VA in CRVO [3, 6]. The evidence for a correlation between change in CST and change in VA for BRVO is likewise mixed. The present study found a weak correlation (r, −0.36 to −0.32) between change in CST and change in VA for BRVO using a fixed-dosing regimen. Hoeh et al. reported a moderate correlation (r, 0.54) [3], while Ou et al. were not able to identify a correlation [7]. Although the results from these studies are varied, to our knowledge, no strong correlations have been found in recent publications in CRVO or BRVO; our investigation supports only weak or moderate correlations. This is consistent with results found in other diseases such as diabetic macular edema (DME) following standardized protocol refractions, visual acuity measurements, and monitored PRN regimens by the DRCR Retina Network [13, 14].
In linear regression analyses of correlation between change in CST and change in BCVA adjusted for baseline factors, only approximately a quarter (COPERNICUS and VIBRANT) to a third (GALILEO) of BCVA changes were attributable to CST changes, and every 100-µm decrease in CST was associated with only approximately a 2-letter gain in visual acuity. In conjunction with the weak to moderate correlations between change in CST and change in VA, these findings do not support the use of CST changes as a surrogate for VA changes in RVO, again similar to analyses done in DME by the DRCR Retina Network [13, 14].
This study has several strengths. This analysis included data from three relatively large randomized clinical trials conducted across multiple sites. Each trial included well-documented and consistent protocols for treatment, imaging, and VA testing. Eyes were included in the analysis only if they had verified BCVA and CST and adhered to the treatment regimen.
Limitations of this study include the post hoc design and thus inability to draw robust conclusions as the analyses was designed after the pre-planned primary, secondary, and exploratory outcomes were known. The analysis also includes CST only, without other qualitative or quantitative OCT or angiographic findings that may improve the predictive power of the linear regression model. To our knowledge, however, studies in other disease such as DME have not shown these other features to improve predictive power substantially beyond that provided by the baseline visual acuity and baseline OCT CST. Studies have reported, for instance, that anatomic features such as DRIL and/or EZ disruption on SD-OCT or the vascular density and foveal avascular zone area on OCT angiography may associate with visual acuity outcomes in eyes with RVO [15,16,17,18], but these studies were not very well controlled or did not involve a large number of patients with standardized protocols or treatment regiments. Additionally, while each trial followed a consistent protocol, differences between the protocols, such as specific treatment regimen, may affect the ability to compare results across trials.
In conclusion, in eyes treated with IAI for macular edema due to retinal vein occlusion, the correlation between change in CST and change in BCVA was weak to moderate for CRVO and weak for BRVO. While changes in CST may be important in determining the need for repeat anti-VEGF to manage macular edema due to retinal vein occlusion, these findings do not support using change in CST as a surrogate for change in visual acuity outcome.
Data availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymised participant data will be considered for sharing once the indication has been approved by major health authorities and if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
References
Kriechbaum K, Michels S, Prager F, Georgopoulos M, Funk M, Geitzenauer W, Schmidt-Erfurth U (2008) Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol 92:518–522. https://doi.org/10.1136/bjo.2007.127282
Hoeh AE, Ach T, Schaal KB, Scheuerle AF, Dithmar S (2009) Long-term follow-up of OCT-guided bevacizumab treatment of macular edema due to retinal vein occlusion. Graefes Arch Clin Exp Ophthal 247:1635. https://doi.org/10.1007/s00417-009-1151-1
Hoeh AE, Ruppenstein M, Ach T, Dithmar S (2010) OCT patterns of macular edema and response to bevacizumab therapy in retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 248:1567–1572. https://doi.org/10.1007/s00417-010-1419-5
Moon CH, Ahn SI, Ohn YH, Kwak HW (2013) Park TK (2013) Visual prognostic value of photopic negative response and optical coherence tomography in central retinal vein occlusion after anti-VEGF treatment. Doc Ophthalmol 126:211–219. https://doi.org/10.1007/s10633-013-9379-9
Fujihara-Mino A, Mitamura Y, Inomoto N, Sano H, Akaiwa K (2016) Semba K (2016) Optical coherence tomography parameters predictive of visual outcome after anti-VEGF therapy for retinal vein occlusion. Clin Ophthalmol 10:1305. https://doi.org/10.2147/OPTH.S110793
Spaide RF, Chang LK, Klancnik JM, Yannuzzi LA, Sorenson J, Slakter JS, Freund KB, Klein R (2008) Prospective study of intravitreal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Am J Ophthalmol 147:298–306. https://doi.org/10.1016/j.ajo.2008.08.016
Ou WC, Brown DM, Payne JF, Wykoff CC (2017) Relationship between visual acuity and retinal thickness during anti–vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol 180:8–17. https://doi.org/10.1016/j.ajo.2017.05.014
Heier J, Clark W, Boyer D, Vitti R, Berliner AJ, Kazmi H, Ma Y, Stemper B, Zeitz O, Sandbrink R, Haller JA (2014) Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology 121:1414–1420. https://doi.org/10.1016/j.ophtha.2014.01.027
Ogura Y, Roider J, Korobelnik J, Holz FG, Simader C, Schmidt-Erfurth U, Vitti R, Berliner AJ, Hiemeyer F, Stemper B, Zeitz O, Sandbrink R, GALILEO StudyGroup (2014) Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am J Ophthalmol 158:1032–1038. https://doi.org/10.1016/j.ajo.2014.07.027
Clark W, Boyer D, Heier J, Brown DM, Haller JA, Vitti R, Kazmi H, Berliner AJ, Erickson K, Chu KW, Soo Y, Cheng Y, Campochiaro PA (2016) Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 123:330–336. https://doi.org/10.1016/j.ophtha.2015.09.035
Schober P, Boer C, Schwarte L (2018) Correlation coefficients: appropriate use and interpretation. Anesth Analg 126:1763–1768. https://doi.org/10.1213/ANE.0000000000002864
Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, Jumper JM, Figueroa M, SCORE Study Investigator Group (2009) SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology 116:504–512. https://doi.org/10.1016/j.ophtha.2008.10.017
Bressler NM, Odia I, Maguire M, Glassman AR, Jampol LM, MacCumber MW, Shah C, Rosberger D, Sun JK, Retina Network DRCR (2019) Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the Protocol T randomized clinical trial. JAMA Ophthalmol 137:977–985. https://doi.org/10.1001/jamaophthalmol.2019.1963
Dugel PU, Campbell JH, Kiss S, Loewenstein A, Shih V, Xu X, Holekamp NM, Augustin AJ, Ho AC, Gonzalez VH, Whitcup SM (2019) Association between early anatomic response to anti-vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of protocol I study data. Retina 39:88–97. https://doi.org/10.1097/IAE.0000000000002110
Samary WA, Shahlaee A, Sridhar J, Khan MA, Ho AC, Hsu J (2016) Quantitative optical coherence tomography angiography features and visual function in eyes with branch retinal vein occlusion. Am J Ophthalmol 166:76–83. https://doi.org/10.1016/j.ajo.2016.03.033
Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O (2017) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol 182:160–167. https://doi.org/10.1016/j.ajo.2017.08.005
Babiuch AS, Han M, Conti FF, Wai K, Silva FQ, Singh RP (2018) Association of disorganization of retinal inner layers with visual acuity response to anti-vascular endothelial growth factor therapy for macular edema secondary to retinal vein occlusion. JAMA Ophthalmol 137(1):38–46. https://doi.org/10.1001/jamaophthalmol.2018.4484
Chan EW, Eldeeb M, Sun V, Thomas D, Omar A, Kapusta MA, Galic IJ, Chen JC (2019) Disorganization of retinal inner layers and ellipsoid zone disruption predict visual outcomes in central retinal vein occlusion. Ophthalmol Retina 3(1):83–92. https://doi.org/10.1016/j.oret.2018.07.008
Funding
This project was funded by the Regeneron Pharmaceuticals, Inc. (Tarrytown, New York) and by philanthropic grants awarded to the Johns Hopkins University School of Medicine (Principal investigator: Dr. Neil Bressler). Regeneron Pharmaceuticals, Inc. participated in design of the study, data analysis, and preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The COPERNICUS, GALILEO, and VIBRANT clinical trials were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. The protocol for each trial was approved by the independent ethics committee or institutional review board at each study site.
Consent to participate
Informed consent was obtained from all individual participants included in the COPERNICUS, GALILEO, and VIBRANT clinical trials.
Conflict of interest
SZG, none; ON, none; SBB, grants to Johns Hopkins University School of Medicine from Amgen, Bayer, Biocon, Biogen, Boehringer-Ingleheim Pharma GmbH & Co., Genentech (Roche), Eyepoint, Mylan Inc, Notal Vision, Novartis, and Regeneron; WD, employment with and stock ownership in Regeneron Pharmaceuticals, Inc.; FA, employment with and stock ownership in Regeneron Pharmaceuticals, Inc.; HM, employment with and stock ownership in Regeneron Pharmaceuticals, Inc.; NMB, grants to Johns Hopkins University from Bayer, Biogen, Genentech (Roche), Novartis, Regeneron, and Samsung Bioepis and contract with AMA as Editor in Chief of JAMA Ophthalmology.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gu, S.Z., Nanegrungsunk, O., Bressler, S.B. et al. Correlation between change in central subfield thickness and change in visual acuity in macular edema due to retinal vein occlusion: post hoc analysis of COPERNICUS, GALILEO, and VIBRANT. Graefes Arch Clin Exp Ophthalmol 260, 3799–3807 (2022). https://doi.org/10.1007/s00417-022-05697-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05697-4