Abstract
Purpose
To compare optical coherence tomography angiography (OCTA) and indocyanine green angiography (ICGA) in terms of reliability in detecting dark halo in patients affected by age-related macular degeneration (AMD) complicated with type 1 macular neovascularization (MNV).
Methods
Eighty-nine eyes of 89 patients were analyzed at the University of Naples Federico II between January 2018 and October 2021. Each patient underwent a complete ophthalmological evaluation including fluorescein angiography, ICGA, spectral domain optical coherence tomography (SD-OCT), and OCTA. OCTA and ICGA images of dark halo were compared. The paired Student’s test and intraclass correlation coefficients were used to evaluate the differences in dark halo measurements between OCTA and ICGA images.
Results
Thirty-six eyes of 36 patients were included in this prospective study. Dark halo area was significantly larger in OCTA than in ICGA (1.49 ± 1.8 mm2 vs. 0.54 ± 0.5 mm2; p = 0.001). Moreover, the agreement between the two types of devices for measuring dark halo areas was poor, with a low intraclass coefficient correlation (0.397).
Conclusion
OCTA could be a useful and necessary tool to investigate dark halo in neovascular AMD due to its ability to visualize the areas of reduced vessel density around MNV in greater detail compared to ICGA.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT05108285
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Age-related macular degeneration (AMD) is a progressive retinal disease that represents the most common cause of legal blindness in developed countries in patients over 55 years of age [1]. AMD complicated with macular neovascularization (MNV) is one of the two advanced forms of AMD, along with geographic atrophy [2, 3].
Until a few years ago, MNV identification was based on the use of both fluorescein (FA) and indocyanine green angiography (ICGA) [4, 5]. ICGA is a consolidated technique used to study choroidal circulation, due to the high binding (98%) of the dye to plasma proteins, and consequently to limit diffusion through choriocapillaris [6].
With the introduction of optical coherence tomography angiography (OCTA), an innovative and non-invasive technique, blood flow in the retina and choriocapillaris can now be analyzed with far greater depth than before [7]. Additionally, OCTA plays a meaningful role in standardizing the current nomenclature for reporting MNV [2].
Although AMD pathogenesis is not yet fully understood, several studies have described the development of MNV, associated with microvasculature choriocapillaris flow deficits in the area surrounding the neovascular lesion [8,9,10]. Rispoli et al. [11] quantified choriocapillaris vascular density changes, renamed dark halo, around MNV before and after anti-vascular endothelial growth factor (VEGF) injections using OCTA. In particular, the authors showed a dark halo fluctuation after intravitreal injections, hypothesizing its role as a biomarker of MNV activity [11].
Dark halo can be detected by either ICGA or OCTA [12, 13]. The former reveals a distinctly dark edge surrounding the MNV until the late stages of the angiography. OCTA detects choriocapillaris flow deficits in the perilesional area, which appear more evident in active MNV [11, 14, 15].
This prospective study aimed to quantify dark halo in patients with AMD complicated with type 1 MNV using ICGA and OCTA and to compare both techniques in terms of reliability in detecting this new activity biomarker.
Materials and methods
During the enrollment period (from January 2018 to October 2021), 89 eyes of 89 subjects affected by exudative AMD were recruited to the Eye Clinic of the University of Naples Federico II.
The criteria for inclusion were age greater than 50 years and diagnosis of treatment-naïve exudative AMD due to the presence of type 1 MNV.
The exclusion criteria were MNV secondary to causes other than AMD, idiopathic polypoidal choroidal vasculopathy (PCV), retinal angiomatous proliferation (RAP), type 2 MNV, previous intravitreal injections of anti-VEGF for CNV, geographic atrophy, subretinal fibrosis, vitreoretinal diseases, retinal vascular diseases, myopia greater than 6 diopters, history of intraocular surgery, and significant lens opacity. We also excluded images with visible eye motion or blinking artifacts and low-quality images obtained with OCTA.
Each patient underwent a complete ophthalmological evaluation, including the evaluation of best-corrected visual acuity (BCVA) according to the Early Treatment of Diabetic Retinopathy Study (ETDRS), slit-lamp biomicroscopy, applanation tonometry, fundus examination, FA, ICGA (Spectralis, Heidelberg Engineering, Heidelberg, Germany), spectral domain (SD)-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany), and OCTA (AngioVue, RTVue XR Avanti, Optovue, Inc., Freemont, CA).
The study was registered on ClinicalTrials.gov (NCT05108285), and all investigations adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patients enrolled in the study.
Optical coherence tomography angiography
OCTA images with the Optovue Angiovue System (software ReVue XR version 2017.1.0.151, Optovue Inc., Fremont, CA, USA) were performed following a standardized protocol based on the split spectrum amplitude decorrelation algorithm (SSADA), as previously described [16].
The AngioAnalyticTM software automatically calculated the vessel density (VD) of the choriocapillaris on a 6 mm × 6 mm macular area. The VD was defined as the percentage area occupied by the microvasculature in the whole scan area and in all sections [17].
The 3D Projection Artifact Removal (PAR) algorithm was performed to improve the quality of OCTA images. For each eye analyzed, the software automatically elaborated the vessel density in the whole scanned area and in all sections of the grid in the choriocapillaris region selected by the operator in retinal angiogram (between upper: Bruch membrane offset − 9 μm and lower: Bruch membrane offset 31 μm).
Excluded from the analysis were images with a signal strength index of less than 80, residual motion artifacts, incorrect segmentation, or low centration or focus.
Dark halo assessment
Dark halo is defined as a choriocapillaris area, surrounding the MNV, characterized by a flow deficit detected by OCTA. The hypofluorescent halo (dark edge of hypofluorescence) around the MNV is also present in the early phase of ICGA and could correspond to the area of dark halo identified by OCTA.
To measure dark halo areas, OCTA and ICGA images were assessed separately, independently by two ophthalmologists (FF and LC). In case of disagreement, a third senior retinal specialist (GC) was asked to evaluate the image.
Firstly, the authors collected and analyzed both choriocapillaris flow density images and scan segmentation by OCTA. The blue space area around the MNV corresponding to dark halo was measured using ImageJ software (Version 1.50i; National Institutes of Health, Bethesda, MD, USA) as previously described [11]. Before the analysis, we used “the set scale” selection tool, entering the known distance and the unit of measurement in pixels to scale the image in millimeter square. The ophthalmologists converted any OCTA images to 8-bit images and then selected the adjust threshold function with the intensity threshold set from 0 to 50 for choriocapillaris OCTA images. The red pixel area around the MNV corresponded to dark halo, and it was manually identified and automatically quantified with the “measure tool” by ImageJ software and collected for statistical analysis.
The readers also collected ICGA images, used for delineation of the dark halo area, taken within 1–4 min after dye injection, according to a previous study [18]. Similar to the analysis performed with OCTA, the ICGA image was manually detected and automatically computed in ImageJ.
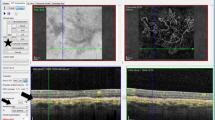
For both OCTA and ICGA images, the whole area, including the MNV and surrounding dark halo, was detected manually and measured automatically using ImageJ. Then, the contour of the MNV area was delineated point by point and measured in millimeter square. On binarized images, the dark halo was calculated as the difference between the whole area and the MNV area (Fig. 1).
Top row. Right eye of a patient with age-related macular degeneration (AMD) complicated with type 1 macular neovascularization (MNV). The whole area (including MNV and dark halo area) and the MNV at indocyanine green angiography (ICGA) were manually selected and automatically measured using ImageJ (A, B). The whole area and the MNV at optical coherence tomography angiography (OCTA) were manually detected and automatically measured using ImageJ (C, D). The difference between the whole area and the MNV corresponds to the dark halo
Statistical analysis
Statistical analysis was performed with SPSS (Version 25 for Windows; SPSS Inc, Chicago, IL, USA). The Shapiro–Wilk test confirmed that all variables were normally distributed. Continuous variables are expressed as mean ± standard deviation (SD). The paired Student’s test was used to evaluate the differences in the dark halo measurements between OCTA and ICGA images. The intraclass correlation coefficients (ICCs) and 95% CIs were used to assess the absolute agreement between the dark halo area measurements from different types of OCTA and ICGA scans. ICC values of < 0.5, between 0.5 and 0.75, between 0.75 and 0.90, and > 0.90 indicated poor, moderate, good, and excellent agreement, respectively. A p-value of < 0.05 was considered statistically significant.
Results
From January 2018 to October 2021, 53 out of 89 eyes were excluded because of poor traditional angiography and OCTA images due to low signal strength. A total of 36 eyes of 36 patients were included in this prospective study (mean age 75 SD ± 10 years; 14 women and 22 men).
From choriocapillary slab OCTA, the mean whole area (MNV + dark halo) was 2.01 SD ± 2.4 mm2, and the MNV area was 0.57 SD ± 1 mm2.
From ICGA scans, the mean whole area (MNV + dark halo) was 1.81 SD ± 2.5 mm2, and the MNV area was 1.27 SD ± 2.1 mm2.
The dark halo areas on OCTA images were statistically significantly larger than those measured at ICGA scans (1.49 SD ± 1.8 mm2 vs. 0.54 SD ± 0.5 mm2; p = 0.001; Table 1; Figs. 2 and 3).
Top row. Right eye of a patient with age-related macular degeneration (AMD) complicated with type 1 macular neovascularization (MNV). Multicolor (A) and infra-red (B) examination showed increased macular thickness associated with drusen and reticular drusen. Autofluorescence image (C) detected a hyper-autofluorescent area in the macular region. Middle row. Fluorescein angiography showed a hyperfluorescent area at the posterior pole (D). Indocyanine green angiography detected a hyperfluorescent vascular network (green line) with a dark halo surrounding (yellow line) (E). Choriocapillaris vessel density map, at the optical coherence tomography angiography (OCTA) scan, showed a wide dark halo around the macular neovascularization (F). Bottom row. Spectral domain optical coherence tomography (SD-OCT) detected drusen, pseudodrusen, and an increased macular thickness associated with subretinal fluid and pigment epithelium detachment in the macular region (G)
Top row. Left eye of a patient with age-related macular degeneration (AMD) complicated with type 1 macular neovascularization (MNV). Multicolor (A) and infra-red (B) examination showed macular hemorrhage with retinal pigment epithelium irregularities. Autofluorescence image (C) detected a hypo/hyper-autofluorescent area in the macular region. Middle row. Fluorescein angiography showed a stippled hyperfluorescence at the posterior pole (D). Indocyanine green angiography detected a hyperfluorescent vascular network (green line) with a dark halo surrounding (yellow line) (E). Vessel density of the choriocapillaris showed dark halo around the macular neovascularization at the optical coherence tomography angiography (OCTA) (F). Bottom row. Spectral domain optical coherence tomography (SD-OCT) detected a wide hyperreflective pigment epithelium detachment, with subretinal and intraretinal fluid (G)
The agreement between the two types of scans for measuring the dark halo areas was poor, with an intraclass correlation coefficient of 0.397 (95% CI: − 0.090 to 0.679).
Discussion
The early identification of activity signs of MNV before they are evident on structural OCT remains a challenge. Nowadays, OCTA plays a crucial role in the diagnosis and management of neovascular AMD due to its reliability in detecting and monitoring retinal and choriocapillaris microvascular changes in affected patients. The proper method of studying type 1 MNV consists of analyzing the neovascular network and its activity signs (including dark halo) at the choriocapillaris segmentation slab. In this study, we first ruled out any artifacts for the two diagnostic techniques to properly quantify the dark halo on OCTA and ICGA as previously described [19].
Although some artifacts in the assessment of dark halo by OCTA have been reported, several studies have observed that its presence is attributable to lower choriocapillaris flow [15, 20]. Jia et al. [15] hypothesized that the area of choriocapillaris ischemia, associated with AMD, may influence the pathogenesis of MNV, which could develop to compensate for the reduced circulation. Moult et al. [20] and Coscas et al. [21] also observed the presence of a halo of choriocapillaris flow deficit located around the MNV on OCTA. These findings were also confirmed by the histologic studies of McLeod et al. [22] and Lutty et al.[23]. The authors described the loss of choriocapillaris around MNV, associated with Bruch’s membrane deposits. They also asserted that changes in the retinal pigment epithelium (RPE), involved in the growth and regression of MNV, could be associated with loss in the choriocapillaris. However, the choriocapillaris flow deficits have been described even in areas with intact RPE, suggesting that loss in choriocapillaris may be the first insult in neovascular AMD [14].
Moreover, according to Seddon et al. [24] who described that histopathologic choriocapillaris changes might influence the production of VEGF causing the growth of the MNV, Rispoli et al. [11] reported dark halo fluctuation after intravitreal injections. The authors suggested that the development of the MNV could induce a decreased flow in the surrounding choriocapillaris due to blood deviation and blood sequestering. Furthermore, they observed that dark halo reached its minimal surface between 6 to 13 days after anti-VEGF injection. In this prospective study, we enrolled naïve exudative AMD patients to detect and quantify the OCTA and ICGA dark halo before the anti-VEGF treatments.
On ICGA, until the late phase of the exam, a sharp dark rim around the MNV was described in a previous study [13]. However, until now, dark halo was observed and described in detail only with OCTA.
Therefore, the goal of our study was to compare the dark halo measurements on OCTA with the ones obtained on ICGA in naïve type 1 MNV in AMD patients in terms of reliability in detecting this new activity biomarker.
The dark halo areas were measured in all eyes with both techniques. Our results showed that the dark halo area was larger on OCTA than ICGA, with poor agreement reflected by the intraclass coefficient correlation. Many causes could explain this finding. First, ICGA analysis of choroidal circulation is performed on the full choroid since the ICGA is a two-dimensional examination, without the possibility of abstracting only the image of the choriocapillaris slab. However, with OCTA, proper segmentation is possible, aiming to study the single plexus and predefined slab that could therefore better highlight the whole area (MNV + dark halo).
Furthermore, OCTA’s greater resolving power and ability to visualize in greater detail the neovascular membrane and areas of reduced VD around the MNV could better clarify our results.
The OCTA larger dark halo area further strengthens the role of OCTA in identifying clinical activity signs of MNV and supports the hypothesis of blood sequestration present during the development of the neovascular network at the expense of the surrounding choriocapillaris areas.
This study suffers from some limitations that should be acknowledged. First of all, we enrolled small number of patients; thus, studies with a larger sample size are needed to confirm these primary results. Moreover, possible error in manually marking the area could not have been avoided.
In the future, the comparison between dark halo changes after anti-VEGF on OCTA and ICGA could be useful to better understand the pathogenesis of neovascular AMD and confirm its key role as an activity biomarker.
Change history
28 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Wong WL, Su X, Li X et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106–e116
Spaide RF, Jaffe GJ, Sarraf D et al (2020) Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 127:616–636
Colijn JM, Buitendijk GHS, Prokofyeva E et al (2017) Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology 124:1753–1763
Gass JD (1987) Pathophysiologic and histopathologic bases for interpretation of fluorescein angiography. In: Stereoscopic atlas of macular diseases: diagnosis and treatment, 3rd edn. St Louis, CV Mosby, pp 19–41
Stanga PE, Lim JI, Hamilton P (2003) Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology 110:15–21
Flower RW (1992) Choroidal angiography today and tomorrow. Retina 12:189–190
Spaide RF, Fujimoto JG, Waheed NK et al (2018) Optical coherence tomography angiography. Prog Retin Eye Res 64:1–55
Ding X, Patel M, Chan CC (2009) Molecular pathology of age-related macular degeneration. Prog Retin Eye Res 28:1–18
Moult EM, Alibhai AY, Rebhun C et al (2020) Spatial distribution of choriocapillaris impairment in eyes with choroidal neovascularization secondary to age-related macular degeneration. Retina 40:428–445
Forte R, Coscas F, Serra R et al (2020) Long-term follow-up of quiescent choroidal neovascularisation associated with age-related macular degeneration or pachychoroid disease. Br J Ophthalmol 104:1057–1063
Rispoli M, Savastano MC, Lumbroso B (2018) Quantitative vascular density changes in choriocapillaris around CNV after anti-VEGF treatment: dark halo. Ophthalmic Sur Lasers Imaging Retina 49:918–924
Scharf JM, Corradetti G, Alagorie AR et al (2020) Choriocapillaris flow deficits and treatment-naïve macular neovascularization secondary to age-related macular Degeneration. Invest Ophthalmol Vis Sci 61:11
Gharbiya M, Pantaleoni FB, Grandinetti F et al (1999) Indocyanine green angiographic findings in idiopathic choroidal neovascularization. Eye (Lond) 13:621–628
Treister AD, Nesper PL, Fayed AE et al (2018) Prevalence of subclinical CNV and choriocapillaris nonperfusion in fellow eyes of unilateral exudative AMD on OCT angiography. Transl Vis Sci Technol 7:19
Jia Y, Bailey ST, Wilson DJ et al (2014) Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 121:1435–1444
Jia Y, Tan O, Tokayer J et al (2012) Split spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 20:4710–4725
Huang D, Jia Y, Gao SS et al (2016) Optical coherence tomography angiography using the Optovue device. Dev Ophthalmol 56:6–12
Corvi F, Cozzi M, Barbolini E et al (2020) Comparison between several optical coherence tomography angiography devices and indocyanine green angiography of choroidal neovascularization. Retina 40:873–880
Coscas G, Lupidi M, Cagini C et al (2018) “False-friend” images on optical coherence tomography angiography: early choroidal neovascularization or artefact? Acta Ophthalmol 96:200–202
Moult E, Choi W, Waheed NK et al (2014) Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina 45:496–505
Coscas GJ, Lupidi M, Coscas F et al (2015) Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina 35:2219–2228
Mc Leod DS, Grebe R, Bhutto I et al (2009) Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci 50:4982–4991
Lutty G, Grunwald J, Majji AB et al (1999) Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis 5:35
Seddon JM, McLeod DS, Bhutto IA et al (2016) Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol 134:1272–1280
Acknowledgements
The manuscript was proofread and edited by Cambridge Proofreading LLC.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fossataro, F., Cennamo, G., Montorio, D. et al. Dark halo, a new biomarker in macular neovascularization: comparison between OCT angiography and ICGA—a pilot prospective study. Graefes Arch Clin Exp Ophthalmol 260, 3205–3211 (2022). https://doi.org/10.1007/s00417-022-05693-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05693-8