Abstract
Background
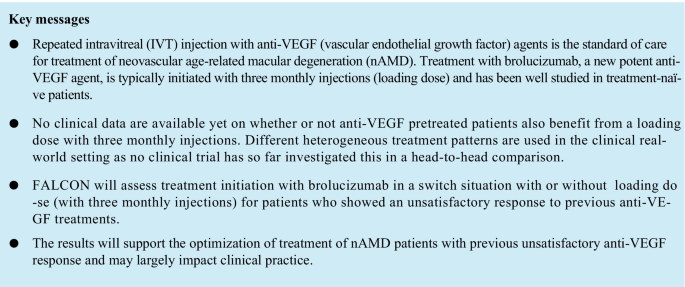
Treatment initiation with brolucizumab, a new potent anti-vascular endothelial growth factor (VEGF) agent, is typically performed with three monthly injections (loading dose) and has been well studied in treatment-naïve patients. However, no clinical data are available yet on whether or not anti-VEGF pretreated patients also benefit from a loading dose. In the clinical setting, different heterogeneous treatment patterns are used as no clinical trial has addressed this so far in a head-to-head comparison. Therefore, the FALCON study is investigating whether patients with unsatisfactory response to previous anti-VEGF treatments benefit from a loading dose at the switch to brolucizumab treatment.
Methods
FALCON is a 52-week, two-arm, randomized, open-label, multicenter, multinational study in patients with residually active neovascular age-related macular degeneration (nAMD) who will be randomized 1:1 and started with brolucizumab 6 mg loading (three monthly loading doses) or brolucizumab 6 mg non-loading (one initial injection) and consecutive treatment every 12 weeks, respectively. The primary objective is to demonstrate non-inferiority of the non-loading vs. loading arm in mean change of best-corrected visual acuity (BCVA) from baseline to the mean value at week 40 to week 52. Secondary objectives include the assessment of anatomical outcomes, treatment intervals, safety and tolerability.
Results
FALCON will be the first study to assess treatment initiation with an anti-VEGF agent in a switch situation with or without loading dose in patients with nAMD.
Conclusions
The results will support the optimization of treatment of patients with previous unsatisfactory anti-VEGF response. Therefore, we expect to see an impact on current clinical practice which has been established for more than a decade.
Trial registration
Clinicaltrials.gov: NCT04679935, date of registration—22-Dec 2020; EUDRACT number: 2019–004763-53, date of registration—03 Dec 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a leading cause of severe vision loss affecting approximately 8.7% of the worldwide population with projected numbers increasing up to 288 million people in 2040 [1].
The neovascular, exudative or wet form of AMD (nAMD) is characterized by retinal vascular leakage and fluid accumulation from neovascularizations. The use of intravitreal (IVT) injection pharmacotherapy targeting vascular endothelial growth factor (VEGF) has shown to improve visual outcomes in nAMD patients [2, 3]. Repeated IVT injection with anti-VEGF agents including the licensed ranibizumab and aflibercept, the non-licensed bevacizumab and the recently approved brolucizumab is used for treatment of nAMD [4, 5].
Brolucizumab is a humanized single-chain antibody fragment (scFv), binding to VEGF-A with high affinity. Its molecular weight of ~ 26 kilodalton allows a delivery of a high molar dose via IVT injection. A 6 mg dose of brolucizumab delivers a molar dose which is approximately 11 and 22 times higher than aflibercept 2 mg and ranibizumab 0.5 mg, respectively [6,7,8].
Brolucizumab was found to be efficacious and safe when compared to aflibercept in treatment-naïve nAMD patients in two large prospective randomized phase 3 trials (HAWK, HARRIER) [9, 10]. Treatment was started with three monthly brolucizumab injections (loading phase), followed by treatment every 12 weeks (q12w) or 8 weeks (q8w). The primary endpoint was met as brolucizumab demonstrated non-inferiority to aflibercept in mean change in best-corrected visual acuity (BCVA) from baseline to week 48 in both trials with more than 50% of patients being maintained on a q12w dosing interval. Brolucizumab also demonstrated superior anatomical outcomes versus aflibercept with fewer patients with intraretinal and/or subretinal fluid (IRF/SRF) and superior reductions in central subfield thickness (CSFT). The visual acuity gains and superior anatomic outcomes were maintained in the second year.
In terms of safety, HAWK and HARRIER described an incidence of intraocular inflammation (IOI) of 4% for brolucizumab 6 mg compared to 1% for aflibercept 2 mg-treated eyes. Most of these cases were reported as mild-to-moderate by the investigators, and the proportion of eyes that lost ≥ 15 letters was comparable between both groups at week 96. It is concluded that brolucizumab exhibited an overall well-tolerated safety profile [9, 10]. Based on the data of the phase 3 trials, brolucizumab has been approved for nAMD treatment in more than 40 countries. In the early post approval phase of brolucizumab use, retinal vasculitis (RV) and/or retinal vascular occlusions (RO), typically in the presence of IOI, were reported. The characterization of these adverse events was included in a safety label update by authorities. Management and treatment recommendations of these adverse events are given in expert consensus statements [11, 12].
Historically, anti-VEGF treatment is typically initiated with three monthly injections (loading dose), followed by a maintenance phase with either fixed (e.g. every 4 or 8 weeks) or individualized treatment intervals, based on treat-and-extend (T&E) or pro re nata (PRN) regimens [13]. Brolucizumab as the latest approved anti-VEGF provides the option to perform a q12w application directly after the loading dose [9, 10]. If, during the course of treatment, an anti-VEGF agent is found to be neither morphologically nor functionally effective without falling below its effective dose, a switch to another anti-VEGF agent may be appropriate. Several studies showed that switching to another anti-VEGF agent might lead to improvement in anatomical parameters and even stabilization or improvement of visual acuity [14,15,16,17]. For these switch patients, treatment is often initiated with a loading dose of three monthly injections, similarly as in treatment-naïve patients. However, no clinical data are available on whether anti-VEGF pretreated nAMD patients also benefit from a loading dose compared with a T&E or PRN regimen following a single injection. Therefore, different heterogeneous treatment patterns are applied in clinical practice as no clinical trial has addressed this so far in a head-to-head comparison.
The FALCON trial will investigate how to optimally initiate the switch to brolucizumab treatment after an unsatisfactory anti-VEGF treatment response and will assess treatment initiation with brolucizumab with or without loading dose. The results will support the optimization of treatment of nAMD patients with previous unsatisfactory anti-VEGF response.
Materials and methods
Study design
FALCON (Clinicaltrials.gov: NCT04679935; EUDRACT number: 2019–004767-53) is a 52-week, two-arm, randomized, open-label, multicenter, multinational study in patients with residually active nAMD. The study consists of three phases (Fig. 1). A screening period of up to 2 weeks will be used to assess eligibility (day − 14 to baseline). At baseline, eligible patients are randomized 1:1 and switched to either brolucizumab 6 mg loading (treatment initiation dose with three monthly initiation doses, referred to as loading in the manuscript) or brolucizumab 6 mg non-loading (one initial injection) and consecutive treatment every 12 weeks, respectively (open-label treatment period, baseline/day 1 to week 48). The last study assessment will be performed at week 52 (post-treatment follow-up period, week 48 to week 52).
During the open-label treatment period, the intended treatment schedule will be every 12 weeks with the option to adjust to an injection every 8 weeks depending on disease activity status. The disease activity decision should be based on the BCVA criterion (BCVA loss ≥ 5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters or ≤ 4 ETDRS letters compared to the previous visit) and/or beyond that on the investigator’s judgment of visual and/or anatomic outcomes and signs of disease activity (e.g. IRF, SRF, hemorrhage, leakage, visual acuity loss over time etc.) The detailed criteria for the disease activity decision are listed in Fig. 1.
Study population
The study population comprises male or female patients ≥ 50 years with active choroidal neovascularization (CNV) secondary to AMD, treated previously for this disease, who showed an unsatisfactory response to previous anti-VEGF treatments. The investigator will assess the eligibility of the patient and the study eye at the screening visit and confirm eligibility prior to randomization. Before enrollment, morphological eligibility will be confirmed by the central reading center as part of the screening period. If both eyes are eligible as per the inclusion and exclusion criteria described below, the eye with the worse visual acuity will be selected for study eye. Patients will be enrolled across two countries: Germany and Switzerland. The key inclusion and exclusion criteria are summarized in Table 1.
Study objectives
FALCON is designed to assess efficacy and safety in patients switched to brolucizumab due to an unsatisfactory response to previous anti-VEGF treatments who will be treated with versus without a loading dose of three monthly intravitreal injections of brolucizumab. The primary objective is to demonstrate non-inferiority of the non-loading vs. loading arm in terms of mean change in best corrected visual acuity (BCVA) from baseline to mean of visits at week 40 to week 52. Secondary objectives include the treatment interval prolongation compared to previous treatment, the functional and anatomical outcomes comparing the two brolucizumab groups and safety and tolerability of brolucizumab treatment.
Data collection
Patients will be enrolled in the study upon signing an informed consent. The screening and baseline visit will be used to assess eligibility and collect baseline characteristics, such as demographic data, medical history and nAMD characteristics. The morphological eligibility will be confirmed by the central reading center. The follow-up visits will take place every 4 weeks, adding up to a total study period of maximum 54 weeks. All visits include an ophthalmic exam as well as intraocular pressure and BCVA measurement. Moreover, spectral domain optical coherence tomography (SD-OCT) images will be taken at every visit. Color fundus photography, fluorescein angiography and optional OCT angiography will be performed at screening and week 52 (end of study visit). All images will be graded independently by the central reading center (CRC) which will be blind to the identity of the treatment arm from the time of randomization until database lock. Patients who discontinue the study treatment will continue in the study with all the scheduled assessments (except administration of study treatment and adherence to prohibited medication list). Those who prematurely withdraw from the study are scheduled for an early discontinuation visit. All patient data will be entered into an electronic case report form (eCRF).
Safety measures
A number of safety precautions are installed to prevent potential serious adverse events. The details on safety precautions and monitoring have been provided to the investigators in the clinical trial protocol. Here, we would like to mention the essentials: First, patients with any active intraocular or periocular infection or active intraocular inflammation will be excluded from study enrollment. Moreover, before every injection, the investigator has to perform a thorough ophthalmic examination including anterior biomicroscopy (slit lamp examination) and posterior segment (indirect fundus) examination; if any signs of intraocular inflammation are present, an injection must not be performed, and the investigators must verify that these conditions are not present in the study eye prior to every injection. In patients developing intraocular inflammation, including retinal vasculitis and/or retinal vascular occlusion, treatment with brolucizumab in the FALCON study should be discontinued, and the events should be promptly managed, and additional images (OCT, FLA and CFP) will be taken. In addition, the patient will be instructed to contact the site immediately for any changes in vision and any symptoms of inflammation. More details on management and treatment recommendation of these adverse events are also provided in expert consensus statements [11, 12].
Outcome measures
The primary efficacy endpoint based on BCVA was chosen to evaluate the benefits of treatment in terms of functional outcome. In addition, anatomical parameters like IRF, SRF and reductions in CSFT will be important outcome measures of treatment efficacy. Detailed objectives of the study are outlined in Table 2.
Statistical analysis
To test the primary efficacy variable, non-inferiority in terms of mean change in BCVA from baseline to mean of visits at week 40 through week 52, a two-sided 95% confidence interval for the treatment difference will be derived from a mixed model for repeated measures (MMRM) with factors treatment arm, baseline BCVA and age. In order to demonstrate non-inferiority, the lower limit of the two-sided 95% confidence interval for the treatment difference (non-loading vs. loading) must be greater than − 4 letters representing the non-inferiority margin.
A sample size of 490 patients (245 patients in each treatment arm) is required to be recruited in the study, so as to have 90% power to demonstrate non-inferiority at a one-sided alpha level of 0.025, assuming equal efficacy and a common standard deviation of 13 letters and accounting for a drop-out rate of ca. 10%.
Results
The FALCON trial will investigate how to optimally initiate a switch to brolucizumab treatment after an unsatisfactory anti-VEGF treatment response including recurrent or recalcitrant macular edema and assesses treatment initiation with an anti-VEGF in a switch situation with or without loading dose. FALCON is to our knowledge the first head-to-head comparison of two different switch regimens and will inform on essential clinical parameters in patients with nAMD and residual disease activity.
The study is planning to recruit patients across approximately 65 centers in Germany and Switzerland. Recruitment is planned to be completed by April 2023; the overall study is expected to be completed by May 2024.
Discussion
Brolucizumab demonstrated non-inferiority to aflibercept in mean BCVA change from baseline to week 48 in treatment-naïve nAMD patients in two large prospective randomized phase 3 trials (HAWK, HARRIER) [9, 10]. In these studies, treatment with brolucizumab was initiated with three monthly injections (loading dose), which is also recommended on the various labels worldwide. If, during the course of treatment, an anti-VEGF agent is found to be neither morphologically nor functionally effective without falling below its effective dose, a switch to another anti-VEGF agent may be appropriate. Some case reports are described where patients were switched from another anti-VEGF to brolucizumab; however, no prospective controlled switch study was reported at time of writing. Moreover, there is no clinical data available on whether anti-VEGF pretreated nAMD patients also benefit from a loading dose, which applies to all currently approved anti-VEGFs. Therefore, different heterogeneous treatment patterns are applied when patients are switched from one anti-VEGF to another as no clinical trial has addressed this so far in a head-to-head comparison. It is known from several clinical studies, that the loading phase plays an important role in short, but also long-term clinical outcomes. Consequently, all historical and currently conducted pivotal trials investigating anti-VEGF treatments in treatment-naïve nAMD patients had a loading phase at trial initiation [18, 19]. A possible hypothesis is that disease activity of treatment-naïve nAMD patients would need to be treated intensively at the beginning to achieve a situation of disease control as quickly as possible. Therewith, further damage of retina cells would be prevented, and long-term visual acuity outcomes would be optimized. Furthermore, there is at least information from real-world data that missing the loading phase in nAMD patients might translate into worse long-term visual outcomes [20].
A recent consensus statement described the central role of fluid accumulation in different anatomical compartments as a biomarker for treatment or re-treatment with an anti-VEGF [21]. In previous clinical trials with shorter acting anti-VEGF agents, a number of patients still showed persistent fluid [22, 23]. In patients who still have persistent fluid as a sign of a residual disease activity, it is questionable whether the loading phase is needed in a switch situation to another anti-VEGF agent. It could be hypothesized that a sub-control situation regarding disease activity is present and just changing to a more potent anti-VEGF agent might be enough to achieve the same visual outcome in comparison to the more intensive treatment with a ‘new start’ with a loading phase. It would be essential to understand if there is a difference between treatment-naïve patients who receive brolucizumab loading doses per approved label and pre-treated nAMD patients after the initiation of brolucizumab (with or without loading doses), be it on visual acuity outcomes or disease control outcomes over time [22, 23].
To our knowledge, FALCON is the first study evaluating the impact of non-loading versus loading in a head-to-head comparison in patients who did show an unsatisfactory response to their previous anti-VEGF treatment. Combining the information on all collected disease parameters, the results will support the optimization of treatment of nAMD patients with an unsatisfactory anti-VEGF response. The study will provide some guidance as to whether a loading dose is required when switching. Moreover, it will show whether brolucizumab can reduce persistent fluid and provides optimized fluid and disease control after the switch. Therefore, we expect an impact on current clinical practice which has been established for more than a decade. As the study addresses a wide range of issues that clinicians face today, its results are awaited with interest. FALCON is taking the first step and paving the way for the investigation of real-world medical questions in a clinical trial setting to support treatment decisions in nAMD patients in routine clinical care.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106-116. https://doi.org/10.1016/S2214-109X(13)70145-1
Bloch SB, Larsen M, Munch IC (2012) Incidence of legal blindness from age-related macular degeneration in denmark: year 2000 to 2010. Am J Ophthalmol 153(209–213):e202. https://doi.org/10.1016/j.ajo.2011.10.016
Campbell JP, Bressler SB, Bressler NM (2012) Impact of availability of anti-vascular endothelial growth factor therapy on visual impairment and blindness due to neovascular age-related macular degeneration. Arch Ophthalmol 130:794–795. https://doi.org/10.1001/archophthalmol.2011.2480
Holz FG, Schmitz-Valckenberg S, Fleckenstein M (2014) Recent developments in the treatment of age-related macular degeneration. J Clin Invest 124:1430–1438. https://doi.org/10.1172/JCI71029
Hussain RM, Ciulla TA (2017) Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin Emerg Drugs 22:235–246. https://doi.org/10.1080/14728214.2017.1362390
Gaudreault J, Gunde T, Floyd HS, Ellis J, Tietz J, Binggeli D, Keller B, Schmidt A, Escher D (2012) Preclinical pharmacology and safety of ESBA1008, a single-chain antibody fragment, investigated as potential treatment for age related macular degeneration. Invest Ophth Vis Sci 53:3025–3025
Tietz J, Spohn G, Schmid G, Konrad J, Jampen S, Maurer P, Schmidt A, Escher D (2015) Affinity and potency of RTH258 (ESBA1008), a novel inhibitor of vascular endothelial growth factor A for the treatment of retinal disorders. Invest Ophthalmol Vis Sci 56:1501
Tadayoni R, Sararols L, Weissgerber G, Verma R, Clemens A, Holz FG (2020) Brolucizumab: a newly developed anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica. https://doi.org/10.1159/000513048
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG, Hawk IHS (2020) HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 127:72–84. https://doi.org/10.1016/j.ophtha.2019.04.017
Dugel PU, Singh RP, Koh A, Ogura Y, Weissgerber G, Gedif K, Jaffe GJ, Tadayoni R, Schmidt-Erfurth U, Holz FG (2021) HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 128:89–99. https://doi.org/10.1016/j.ophtha.2020.06.028
Baumal CR, Bodaghi B, Singer M, Tanzer DJ, Seres A, Joshi MR, Feltgen N, Gale R (2020) Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. https://doi.org/10.1016/j.oret.2020.09.020
Holz FG, Heinz C, Wolf A, Hoerauf H, Pleyer U (2021) Intraocular inflammation with brolucizumab use: patient management-diagnosis-therapy. Ophthalmologe 118:248–256. https://doi.org/10.1007/s00347-021-01321-8
Wykoff CC, Clark WL, Nielsen JS, Brill JV, Greene LS, Heggen CL (2018) Optimizing Anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J Manag Care Spec Pharm 24:S3–S15. https://doi.org/10.18553/jmcp.2018.24.2-a.s3
Gale RP, Pearce I, Eter N, Ghanchi F, Holz FG, Schmitz-Valckenberg S, Balaskas K, Burton BJL, Downes SM, Eleftheriadis H, George S, Gilmour D, Hamilton R, Lotery AJ, Patel N, Prakash P, Santiago C, Thomas S, Varma D, Walters G, Williams M, Wolf A, Zakri RH, Igwe F, Ayan F (2020) Anatomical and functional outcomes following switching from aflibercept to ranibizumab in neovascular age-related macular degeneration in Europe: SAFARI study. Br J Ophthalmol 104:493–499. https://doi.org/10.1136/bjophthalmol-2019-314251
Pikkel J, Attas S (2018) “What should I inject next?” Challenging treatment decisions in the multiple anti-VEGF: a review of publications exploring anti-VEGF switching for nAMD. Int Ophthalmol 38:2031–2039. https://doi.org/10.1007/s10792-017-0695-z
Seguin-Greenstein S, Lightman S, Tomkins-Netzer O (2016) A meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age-related macular degeneration following switching to treatment with aflibercept. J Ophthalmol 2016:4095852. https://doi.org/10.1155/2016/4095852
Marquis LM, Mantel I (2020) Beneficial switch from aflibercept to ranibizumab for the treatment of refractory neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 258:1591–1596. https://doi.org/10.1007/s00417-020-04730-8
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. https://doi.org/10.1056/NEJMoa054481
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, View, Groups VS (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006
Sagkriotis A, Chakravarthy U, Griner R, Doyle O, Wintermantel T, Clemens A (2021) Application of machine learning methods to bridge the gap between non-interventional studies and randomized controlled trials in ophthalmic patients with neovascular age-related macular degeneration. Contemp Clin Trials 104:106364. https://doi.org/10.1016/j.cct.2021.106364
Kodjikian L, Parravano M, Clemens A, Dolz-Marco R, Holz FG, Munk MR, Nicolo M, Ricci F, Silva R, Talks SJ, Verma RK, Zarranz-Ventura J, Zweifel SA (2021) Fluid as a critical biomarker in neovascular age-related macular degeneration management: literature review and consensus recommendations. Eye (Lond). https://doi.org/10.1038/s41433-021-01487-0
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, Investigators A (2020) Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: A Randomized Controlled Trial. Adv Ther 37:1173–1187. https://doi.org/10.1007/s12325-020-01236-x
Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J, Group TS (2018) Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology 125:57–65. https://doi.org/10.1016/j.ophtha.2017.07.014
Acknowledgements
We thank Martina Junge and Jessica Voegeler, who contributed to the study idea and critically reviewed the study proposal. We thank the FALCON study group, all sites and investigators, for their participation in the study.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study is funded by the Novartis Pharma GmbH, Nuernberg, Germany.
Author information
Authors and Affiliations
Contributions
FH is the principal coordinating investigator of the trial. SS, AW, HA, KL, AP, NF, RG, CQ, AC and KJ participated in the study design/implementation/conduct of the FALCON study. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The clinical study was designed and is conducted in accordance with the ethical principles laid down in the Declaration of Helsinki. The study has been approved by the ethical committee of the medical faculty of the University of Bonn.
Consent to participate
Written informed consent was obtained from all participants prior to study inclusion.
Consent for publication
Not applicable.
Conflict of interest
FH, SS, AW, KL, AP, NF and RG received investigator fees from Novartis. AC is full time employee and shareholder of Novartis Pharma AG. CQ and KJ are full-time employees of Novartis Pharma GmbH.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holz, F.G., Schmitz-Valckenberg, S., Wolf, A. et al. A randomized, open-label, multicenter study of switching to brolucizumab with or without a loading dose for patients with suboptimal anatomically controlled neovascular age-related macular degeneration—the FALCON study. Graefes Arch Clin Exp Ophthalmol 260, 2695–2702 (2022). https://doi.org/10.1007/s00417-022-05591-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05591-z