Abstract
Background
Benzalkonium chloride (BAK), the most commonly used preservative in anti-glaucoma eye drops, inflicts damage to the ocular surface. A novel anti-glaucoma formulation that avoids the use of BAK has been developed. The aim of this study was to evaluate the cytotoxicity of this formulation and to compare it with an ophthalmic solution containing BAK.
Methods
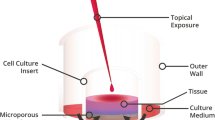
Two different latanoprost eye drops were used: one ophthalmic solution (LSc) containing BAK 0.02% and one ophthalmic nanoemulsion (LNe) with a soft preservative (potassium sorbate 0.18%). Human epithelial conjunctival cells were incubated for 15, 30, and 60 min with either LSc or LNe. The cytotoxicity was determined by MTT assay. Cell death was measured by flow cytometry using annexin V–FITC and propidium iodide.
Results
The values of cell viability and proliferation obtained from cells exposed to LNe were between 80 and 90% relative to the control group, whereas values obtained from cells exposed to LSc were around 30% at all study times (p < 0.05 at 15 and 30 min; p < 0.01 at 60 min). The percentage of viable cells decreased significantly when cells were incubated with LSc compared with cells incubated with LNe at all the study times, while the percentage of cells in late apoptosis/necrosis increased significantly in cells exposed to LSc compared to LNe.
Conclusions
The new latanoprost nanoemulsion is significantly less cytotoxic on human conjunctival cells than LSc. These results suggest that the new formulation might be gentler on the eye surface than currently available BAK-preserved latanoprost solutions.


Similar content being viewed by others
Data availability
Data is available anytime upon request to the corresponding author. Data is stored by investigators at Laboratory of Ocular Research, Pathology Department, Faculty of Medicine, Buenos Aires University.
References
Grzybowski A, Och M, Kanclerz P, Leffler C, Moraes CG (2020) Primary open angle glaucoma and vascular risk factors: a review of population based studies from 1990 and 2019. J Clin Med 9(3):761. https://doi.org/10.3390/jcm9030761
Sharif NA (2018) Glaucomatous optic neuropathy treatment options: the promise of novel therapeutics, techniques and tools to help preserve vision. Neural Regen Res 13(7):1145–1150. https://doi.org/10.4103/1673-5374.235017
Stein JD, Khawaja AP, Weizer JS (2021) Glaucoma in adults-screening, diagnosis, and management: a Review. JAMA 325(2):164–174. https://doi.org/10.1001/jama.2020.21899
Schuster AK, Erb C, Hoffmann EM, Dietlein T, Pfeiffer N (2020) The diagnosis and treatment of glaucoma. Dtsch Arztebl Int 117(13):225–234. https://doi.org/10.3238/arztebl.2020.0225
Goto Y, Ibaraki N, Miyake K (2003) Human lens epithelial cell damage and stimulation of their secretion of chemical mediators by benzalkonium chloride rather than latanoprost and timolol. Arch Ophthalmol 121(6):835–839. https://doi.org/10.1001/archopht.121.6.835
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F (2010) Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res 29(4):312–334. https://doi.org/10.1016/j.preteyeres.2010.03.001
Steven DW, Alaghband P, Lim KS (2018) Preservatives in glaucoma medication. Br J Ophthalmol 102(11):1497–1503. https://doi.org/10.1136/bjophthalmol-2017-311544
Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM (2016) Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol 12(11):1279–1289. https://doi.org/10.1080/17425255.2016.1209481
Sedlak L, Wojnar W, Zych M, Wyględowska-Promieńska D (2020) Influence of timolol, benzalkonium-preserved timolol, and benzalkonium-preserved brimonidine on oxidative stress biomarkers in the tear film. Cutan Ocul Toxicol 39(3):260–268. https://doi.org/10.1080/15569527.2020.1787435
Datta S, Baudouin C, Brignole-Baudouin F, Denoyer A, Cortopassi GA (2017) The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest Ophthalmol Vis Sci 58(4):2406–2412. https://doi.org/10.1167/iovs.16-20903
Galletti JG, Gabelloni ML, Morande PE, Sabbione F, Vermeulen ME, Treani AS, Giordano MS (2013) Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol 6(1):24–34. https://doi.org/10.1038/mi.2012.44
Guzmán M, Sabbione F, Gabelloni ML, Vanzulli S, Trevani AS, Giordano MN, Galleti JG (2014) Restoring conjunctival tolerance by topical nuclear factor-κB inhibitors reduces preservative-facilitated allergic conjunctivitis in mice. Invest Ophthalmol Vis Sci 55(9):6116–6126. https://doi.org/10.1167/iovs.14-14075
Daull P, Raymond E, Feraille L, Garrigue JS (2018) Safety and tolerability of overdosed artificial tears by Abraded Rabbit Corneas. J Ocul Pharmacol Ther 34(10):670–676. https://doi.org/10.1089/jop.2018.0040
Murugesan V, Dwivedi R, Saini M, Gupta V, Dada T, Vivekanandhan S (2021) Tear neuromediators in eyes on chronic topical antiglaucoma therapy with and without BAK preservatives. Br J Ophthalmol 105(1):141–148. https://doi.org/10.1136/bjophthalmol-2019-314234
Kabashima K, Murakami A, Ebihara N (2020) Effects of benzalkonium chloride and preservative-free composition on the corneal epithelium cells. J Ocul Pharmacol Ther 36(9):672–678. https://doi.org/10.1089/jop.2019.0165
Baudouin C (2008) Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol 86(7):716–726. https://doi.org/10.1111/j.1755-3768.2008.01250.x
Kaštelan S, Tomić M, MetežSoldo K, Salopek-Rabatić J (2013) How ocular surface disease impacts the glaucoma treatment outcome. Biomed Res Int 2013:696328. https://doi.org/10.1155/2013/696328
Vélez-Gómez MC, Vásquez-Trespalacios EM (2018) Adherence to topical treatment of glaucoma, risk and protective factors: a review. Arch Soc Esp Oftalmol 93(2):87–92. https://doi.org/10.1016/j.oftal.2017.07.012
Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, Kim T, Mehta JS, Messmer EM, Pepose JS, Sangwan VS, Weiner AL, Wilson SE, Wolffsohn JS (2017) TFOS DEWS II iatrogenic report. Ocul Surf 15(3):511–38. https://doi.org/10.1016/j.jtos.2017.05.004
Fogagnolo P, Torregrossa G, Tranchina L, Ferreras A, De Cillá S, Labbé A, Figus M, Ottobelli L, Rosetti L (2019) Tear film osmolarity, ocular surface disease and glaucoma: a review. Curr Med Chem 26(22):4241–4252. https://doi.org/10.2174/0929867326666190725160621
Diebold Y, Calonge M, De Salamanca AE, Callejo S, Corrales RM, Sáez V, Siemasko KF, Stern ME (2003) Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Investig Ophthalmol Vis Sci 44(10):4263–74. https://doi.org/10.1167/iovs.03-0560
Nozhat Z, Khalaji MS, Hedayati M, Kia SK (2020) Different methods for cell viability and proliferation assay: essential tools in pharmaceutical studies. Anticancer Agents Med Chem. https://doi.org/10.2174/1871520621999201230202614
Walsh K, Jones L (2019) The use of preservatives in dry eye drops. Clin Ophthalmol 13:1409–1425. https://doi.org/10.2147/OPTH.S211611
Yanochko GM, Khoh-Reiter S, Evans MG, Jessen BA (2010) Comparison of preservative-induced toxicity on monolayer and stratified Chang conjunctival cells. Toxicol In Vitro 24(4):1324–1331. https://doi.org/10.1016/j.tiv.2010.02.001
Stewart WC, Oehler JC, Choplin NT, Markoff JI, Moster MR, Ichhpujani P, Nelson LA (2013) A comfort survey of timolol hemihydrate 0.5% solution once or twice daily vs timolol maleate in sorbate. J Curr Glaucoma Pract 7(1):11–16. https://doi.org/10.5005/jp-journals-10008-1130
Khiev D, Mohamed ZA, Vichare R, Paulson R, Bhatia S, Mohapatra S, Lobo GP, Valapala M, Kerur N, Passaglia CL, Mohapatra SS, Biswal MR (2021) Emerging nano-formulations and nanomedicines applications for ocular drug delivery. Nanomaterials (Basel) 11(1):173. https://doi.org/10.3390/nano11010173
Weng Y, Liu J, Jin S, Gup W, Liang X, Hu Z (2017) Nanotechnology-based strategies for treatment of ocular disease. Acta Pharmaceutica Sinica B 7(3):281–291
Casiraghi JF, Grigera D, Peyret AJ, del Papa M, Passerini MS.Re:GEN Open.Jun 2021.110–116. https://doi.org/10.1089/regen.2021.0018
Brasnu E, Brignole-Baudouin F, Riancho L, Warnet JM, Baudouin C (2008) Comparative study on the cytotoxic effects of benzalkonium chloride on the Wong-Kilbourne derivative of Chang conjunctival and IOBA-NHC cell lines. Mol Vis 14:394–402
Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun JM, Warnet JM, Baudouin C (2008) In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res 33(4):303–312. https://doi.org/10.1080/02713680801971857
Pellinen P, Huhtala A, Tolonen A, Lokkila J, Mäenpää J, Uusitalo H (2012) The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr Eye Res 37(2):145–154. https://doi.org/10.3109/02713683.2011.626909
Labbé A, Gabison E, Brignole-Baudouin F, Riancho L, Menashi S, Baudouin C (2015) Increased extracellular matrix metalloproteinase inducer (EMMPRIN) expression in the conjunctival epithelium exposed to antiglaucoma treatments. Curr Eye Res 40(1):40–47. https://doi.org/10.3109/02713683.2014.915574
Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F (2005) In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 46(12):4594–4599. https://doi.org/10.1167/iovs.05-0776
Acknowledgements
Rodrigo M. Torres, MD PhD, for his scientific advice.
Funding
This work was supported by Poen Laboratories S.A.U.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tau, J., Passerini, M.S., del Papa, M. et al. A novel ophthalmic latanoprost 0.005% nanoemulsion: a cytotoxicity study. Graefes Arch Clin Exp Ophthalmol 260, 1941–1946 (2022). https://doi.org/10.1007/s00417-021-05536-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05536-y