Abstract
Introduction
Corneal epithelial toxicity and delayed healing process have already been attributed to preservatives or some excipients. We study the effects of galenic components in antiglaucoma drugs such as benzalkonium chloride (BAC) or surfactants like macrogolglycerol hydroxystearate 40 (MGHS 40) on corneal toxicity in an ex vivo system mimicking chronic use.
Methods
Latanoprost-containing eyedrops are available with and without preservatives on the market. Unpreserved, they are available in different formulations with various excipients like MGHS at different concentrations (0%, 2.5%, and 5%). We studied these in the ex vivo bioreactor (EVEIT) on initially injured rabbit corneas. The drugs were applied six times daily for observation periods of 3 or 5 days. BAC, 5% MGHS 40 solution, and 0.18% hyaluronic acid served as controls. Macroscopic photographic, biochemical methods and corneal integrity quantification were used for evaluation. Toxicity was assessed by measuring wound healing and corneal fluorescein sodium permeability and was confirmed by histology studies.
Results
The BAC-preserved formulation resulted in high corneal toxicity, which was expected. Interestingly, the preservative-free (PF) formulation containing 5% MGHS 40, carbomer, macrogol 4000, and sorbitol showed the highest corneal toxicity, followed by the control formulation with equal MGHS 40 concentration, which presented significantly less damage. No toxicity was shown by eyedrops containing 2.5% MGHS 40 or salts only.
Conclusion
Our study demonstrates a significant corneal toxicity of certain formulations of PF antiglaucoma ophthalmic drugs containing 5% MGHS 40 with other excipients compared to other formulations with lower MGHS 40 concentrations (2.5% or 0%), or even compared to the solution containing 5% MGHS alone. This suggests a concentration-dependent toxicity of MGHS 40, especially in interaction with other excipients, which may increase its epithelial toxicity, and that has to be considered in clinical glaucoma therapy. Further single-component formulation trials are needed to support this interpretation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Since preserved and unpreserved latanoprost-containing ophthalmic drugs, belonging to first-line therapy of glaucoma treatment, are often administered over many years, questions naturally arise about the associated problems of tolerability and ocular surface toxicity. |
The toxic effect of preservatives on the ocular surface has been recognized for several years and demonstrated through several studies, but little attention has been paid to the other excipients and their interactions. |
In vivo tolerability is time-dependent; therefore, either long-term studies or mimicking toxicity models are needed. The ex vivo bioreactor (EVEIT) model has been assessed in further studies and is relevant for our investigation. |
The purpose of our study was to investigate the overall effect of preserved and unpreserved 0.005% latanoprost antiglaucoma drugs with different macrogolglycerol hydroxystearate 40 (MGHS 40) concentrations (0%, 2.5%, and 5%) on the corneal healing rate as well as on tissue integrity after mimicking chronic administration. |
What was learned from the study? |
Our first study over 3 days showed clear toxic effects of preservatives such as benzalkonium chloride (BAC) as well as evidence of slight changes in epithelial healing between the different unpreserved latanoprost formulations. |
The toxic effect could need a longer time period of testing to become apparent and significant. |
Our second study over 5 days confirmed that the toxic effect of MGHS 40 on the cornea is concentration-dependent and also enhanced by interactions with other excipients. |
The association of MGHS in high concentration (5%) with viscosity agents such as macrogol 4000 or carbomer appears to be of great concern for ocular surface health in long-term glaucoma treatment. |
Introduction
The goal in the treatment of glaucoma is to maintain the patient’s visual function and quality of life by reducing intraocular pressure. Before surgical measures and in addition to some therapy-accompanying procedures, a topical medication with eyedrops is applied. Failure to take medications can be the result of poor local tolerance and can result in progressive damage to the optic nerve and eventual vision loss in patients with glaucoma. Unless contraindicated, the latest European Glaucoma Society guidelines recommend the use of β-blockers or prostaglandin analogues as first-line therapy [1]. Most patients with glaucoma require the use of hypotensive drops for years, leading to an increased risk of developing ocular surface disease. Moreover, increased preoperative exposure to ophthalmic solutions preserved with benzalkonium chloride (BAC) is a risk factor for earlier surgical failure [2]. Therefore, the toxic effects of preservatives in eyedrops have been investigated in numerous studies and it was recommended that ideally, future antiglaucoma therapy should become 100% preservative free (PF), in order to minimize therapy failure and ocular toxicity [3]. However, little attention has been paid to the other excipients in the drug formulation and their interactions with the ocular surface. Galenic formulation of a drug is based on the combination of the active ingredient with additives and excipients with the aim of optimizing the formulation in terms of physicochemical stability, retention time, and tolerability. Furthermore, these excipients are able to influence the pharmacokinetic profile, effectiveness, and acceptability of the finished product. Here, the aim was to investigate the influence of excipients such as macrogolglycerol hydroxystearate 40 (MGHS 40), a non-ionic surfactant, on possible undesired ocular surface interactions. While some cell culture experiments did not show acute effects from MGHS 40 [4], other comparative studies did show inflammatory and cytotoxic responses correlated with the concentration of this excipient [5]. Four commercially available drugs containing latanopost were tested in this study for their healing impact and corneal toxicity over 3 and 5 days in an ex vivo model of the anterior segment of the eye, the Ex Vivo Eye Irritation Test (EVEIT). This test system was established according to the 3R principles [6]—reduction, refinement, and replacement—of common animal experiments. EVEIT was used as a model for corneas under local medication to test the impairment of corneal healing as well as toxic effects of corneal drugs in short- and long-term periods [7, 8]. EVEIT, as an animal-free alternative to the Draize test, allows research in the field of healing, acute and chronic toxicity, pharmacology, and pharmacokinetics. It is characterized by a high sensitivity and reproducibility, since test substances can be applied at any frequency in a very controlled manner; moreover their effect can be flexibly documented in terms of corneal vitality, superficial as well as deep tissue changes or permeation [7,8,9,10]. Our study is divided into two observation periods: in the first study over 3 days, we tested BAC-preserved versus PF latanoprost formulations in rabbit corneas with regard to their toxicity. Only PF eyedrops containing latanoprost with different excipients were analyzed for their toxicities in the follow-up study with 5 days of observation.
Methods
Test Substances
The influences of preserved and PF latanoprost (50 µg/ml)-containing eyedrops on the market were firstly investigated in a study over 3 days on the efficacy of corneal healing. Based on the findings of this study, a follow-up study with prolonged exposure and observation time was conducted with PF latanoprost preparations only. For details of the latanoprost formulations, please refer to Table 1.
For the 3-day study, we tested all formulations compared to 0.02% BAC and to PF hyaluronic acid 0.18% eyedrops (control reference; CT). For the 5-day study, only the PF eyedrops (formulations A, B and C) were examined. They include different MGHS concentrations with 5% being the highest. Thus, a single-component 5% MGHS 40 solution was used as a control diluted with distilled water + NaCl. The CT was also used as the control reference.
All test substances were applied directly by dripping a volume of 30–50 µl six times daily onto the apex of controlled epithelial injured corneas. This high frequency application model allows one to mimic the chronic use of treatment in long-term diseases such as glaucoma. The experimental approaches were carried out in triplicate (3-day study) or quintuplicate (5-day study). The osmotic pressure (Osmomat 3000, Gonotec, Berlin, Germany) and the pH (pH 1000 H, pHenomenal®, VWR, Germany) of all used formulations were measured before the treatments. These were in the physiological range around pH 6–7 and around 300 mosmol/kg, except for the hypoosmotic control substance CT (162 mosmol/kg).
Ex Vivo Eye Irritation Test (EVEIT)
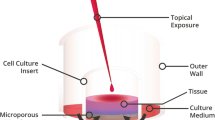
The EVEIT system is a non-animal-consuming test system that simulates the anterior ocular chamber with a physiological corneal barrier for studying corneal drug toxicity and permeability. This system has been previously described in detail [7, 8]. Approval by an ethics committee was not required for the experiments, as the eyes came from slaughtered animals. No live animal or human testing was involved in this work. No human tissues were involved and no patient data were used. Animal tissues involved were procured as waste product from the food industry which falls under the purview of the governmental German Veterinary Office. Briefly, rabbit corneal tissues (including the limbus region) are isolated and placed into a specialized bioreactor chamber. The rabbit corneas are prepared and cultivated within 8 h post mortem. For nutrition, the chamber is constantly supplied with a culture medium containing Earle’s salts (Minimal Essential Medium Eagle, PAN-Biotech, Germany) + HEPES buffer 5.8 g/l. In these experiments, the medium was constantly replenished using a micropump (Ismatec IPC, IDEX Health & Science GmbH, Wertheim, Germany) with an entrance pH value of 7.4 and a flow rate of 6.44 µl/min, which imitates the physiological conditions in the eye. The corneas were incubated at a temperature of 32 °C and a humidity of more than 95%. All the experiments were performed in accordance with the Code of Ethics of the World Medical Association.
Initial Corneal Defects
In the first EVEIT experiment, formulations were tested on three corneas each, based on previously published results that used the same model [11]. For the second experiment, the number of tested corneas was increased to five per product in order to increase the statistical significance of the results and to make sure they were homogenous. After 24 h of stabilization within the EVEIT culturing system, the corneas were evaluated by microscopy. Corneas with epithelial defects or opacities were excluded from further experiments. During the experiments, the integrity of both the epithelial and endothelial sides was monitored daily using a phase contrast microscope-integrated camera (KY-F1030U Camera, JVC, Bad Vilbel, Z16 APO Microscope, Wetzlar, Germany) connected to DISKUS software (Hilgers, Koenigswinter, Germany). Before corneal healing experiments started, four small corneal abrasions measuring 2.9 ± 0.9 mm2 were induced on each cornea by an abrasive drill, which was placed on the cornea in a square pattern. Defect sizes were monitored by fluorescein sodium stains (0.17% an aqueous solution), with yellow-green fluorescence indicating the areas of epithelial defects which were circumscribed using the DISKUS software tool. The erosion sizes (sum of four erosions of each cornea) are given in square millimeters.
Analytics
Epithelial Surface Imaging
Daily epithelial surface monitoring with and without fluorescein staining occurred using a phase contrast microscope-integrated camera. Epithelial defects were detected by fluorescein sodium stains with yellow-green fluorescence and were quantified by using the connected DISKUS software tool.
Corneal Barrier Function
Corneal fluorescein sodium permeability testing is an indicator of the integrity of the corneal barrier function. Tests were carried out on day 0 and finally after the experimental time. In this regard, a PF solution of fluorescein sodium (AppliChem GmbH, 5 mg/ml, 100 μl per cornea) was applied on the apex of the corneas for 60 s, rinsed with Ringer’s solution, and the fluorescein concentration was measured in the perfusion medium of the anterior chamber via photometry (SpectraMax iD3, MolecularDevices) after further 60 min incubation (32 °C, 95% humidity).
Metabolic Activity
Corneal vitality was assessed by demonstrating metabolic activity. Therefore, the concentrations of glucose (Glucose GOD FS, DiaSys Diagnostic Systems GmbH, Holzheim, Germany) and lactate (Lactate FS, DiaSys Diagnostic Systems GmbH, Holzheim, Germany) were quantified photometrically (EPOCH microplate reader, BioTek Instruments GmbH, Bad Friedrichshall, Germany) in the eluted medium of the anterior chamber after bypassing the corneal endothelium. Low glucose levels indicate consumption of glucose contained in the cell culture medium; conversely, high lactate levels indicate energy consumption. Thus high glucose levels with low lactate levels indicate low energy consumption or cell death as result of toxic action. The glucose/lactate concentrations were analyzed daily.
Histology
Finally, corneal morphology was histologically evaluated using a standard hematoxylin and eosin staining method.
Statistical Methods
The statistical analysis of corneal erosions and of glucose/lactate concentrations was performed using Student’s t test. Results were presented as mean ± standard deviation (SD) and numbers. A p value of less than 0.05 was considered statistically significant.
Results
Corneal Healing
In both the 3-day and follow-up 5-day studies, fast and persistent healing was observed from day 1 for the control reference CT, completed by day 2, closely followed by latanoprost formulations A and B with stable epithelial closure at day 3 (Figs. 1 and 2). This was observed in all tested corneas (n = 3 for the 3-day study and n = 5 for the 5-day study). After initial healing, the control 0.02% BAC and BAC-preserved latanoprost formulation X showed strong epithelial reopening from day 2 to day 3 (Fig. 1). Group C showed delayed healing at day 2, not completed at day 3 (Fig. 1) and significant reopening of the lesions in all five tested corneas with a 156% increase in lesion size compared to baseline (p < 0.05) after a 5-day observation period (Fig. 2). For the corneas treated with MGHS 5% alone (Fig. 2), different healing processes were observed throughout the study period, ranging from complete and stable healing (Fig. 2, “MGHS 5% 1”) to delayed healing with residual lesions and up to a 65% increase in lesion size compared to baseline for one cornea (Fig. 2, “MGHS 5% 2”).
Microscopic evaluation of the epithelial healing process of the fluorescein stained corneas during 3 days observation period (n = 3 corneas per test item). Healing was observed from day 1 for the control reference CT, completed by day 2, closely followed by latanoprost formulations A and B with stable epithelial closure at day 3. After initial healing, the control 0.02% BAC and BAC-preserved latanoprost formulation X showed strong epithelial reopening from day 2 to day 3. Group C showed delayed healing at day 2, not completed at day 3. BAC benzalkonium chloride, MGHS macrogolglycerol hydroxystearate 40
Representative fluorescein-stained microscopical images of the corneal healing process during experimental period of 5 days with drug application six times daily (n = 5 corneas per test item). All five corneas per test item are shown in detail in Figs. S1–S5 in the supplementary material. Healing was observed from day 1 for the control reference CT, completed by day 2, closely followed by latanoprost formulations A and B with stable epithelial closure at day 3. Group C showed delayed healing at day 2, not completed at day 3 and significant reopening of the lesions up to day 5. For the corneas treated with MGHS 5% alone, different healing processes were observed throughout the study period, ranging from complete and stable healing (MGHS 5% 1) to delayed healing with residual lesions and an increase in lesion size compared to baseline (MGHS 5% 2). MGHS macrogolglycerol hydroxystearate 40
There is a final significant (p < 0.05) reopening of the epithelium compared to baseline for formulations X and C and the control 0.02% BAC (Fig. 3).
Graphical illustration of the corneal healing process under six times daily drug application over 3 days (a) with n = 3 corneas or 5 days (b) with n = 5 corneas per test item. The mean corneal erosion sizes in mm2 are plotted against time (experimental days). We observe a final significant (p < 0.05) reopening of the epithelium compared to baseline for formulations X and C and the control 0.02% BAC. BAC benzalkonium chloride, MGHS macrogolglycerol hydroxystearate 40
Corneal Barrier Function After Test Substance Application
Investigations for corneal integrity quantification were carried out on day 0 and at the final day after exposure to the test substances six times daily. This occurred for both the 3-day study and the subsequent 5-day study. The difference between final day minus day 0, the “delta” (Δ), was determined to compare the effect of formulations on the cornea. This is shown in Figs. 4 and 5.
Corneal integrity changes of all corneas (n = 3 each test item) after 3 experimental days of fluorescein sodium permeation through the cornea. Fluorescein permeability Δ value was significantly higher for BAC-preserved latanoprost formulation X and the control 0.02% BAC (p < 0.05) than for A, B, C, and CT. No significant difference was seen between formulation X and BAC. BAC benzalkonium chloride
Corneal integrity changes of all corneas (n = 5 each test item) after 5 experimental days of fluorescein sodium permeation through the cornea. The ΔD5–D0 values were significantly higher for C compared to A, B, and MHGS 5% (p < 0.05) highlighting a reduction in corneal integrity due to test substance application six times daily. No statistical difference was observed between the ΔD5–D0 values of A, B, and CT. MGHS macrogolglycerol hydroxystearate 40
Delta analysis of day 3 minus day 0 (ΔD3–D0) (Fig. 4) shows clear and high differences in corneal barrier function. Fluorescein permeability Δ value was significantly higher for BAC-preserved latanoprost formulation X and the control 0.02% BAC (p < 0.05) than for A, B, C, and CT. No significant difference was seen between formulation X and BAC.
For the 5-day study (Fig. 5), the ΔD5–D0 values were significantly higher for C compared to A, B, and MHGS 5% (p < 0.05) highlighting a reduction in corneal integrity due to test substance application six times daily. No statistical difference was observed between the ΔD5–D0 values of A, B, and CT.
Corneal Metabolism
Daily quantification of the metabolic activity of each cornea was performed to evaluate metabolic stress due to test substance application during long-term observation over 3 days (Fig. 6a, b) or 5 days (Fig. 6c, d). There is an increase in lactate metabolism measured for C, X, and 0.02% BAC control, but lactate values only significantly differed (p < 0.05) between day 3 and baseline for formulation X (Fig. 6a). For the 5-day testing, lactate levels remained in physiological range, with a significant increase for C and MGHS 5% (p < 0.05) compared to CT (Fig. 6c). In both studies, glucose levels remained in physiological range (Fig. 6b, d).
Graphical analysis of the daily measured lactate and glucose values as an indicator of metabolic activity during test substance application over 3 days (a, b) or 5 days (c, d). Standard deviations are integrated. There is an increase in lactate metabolism measured for C, X, and 0.02% BAC control, but only for formulation X were latanoprost values significantly different (p < 0.05) at day 3 compared to baseline (a). For the 5-day testing, lactate levels remained in physiological range, with a significant increase for C and MGHS 5% (p < 0.05) compared to CT (c). In both studies, glucose levels remained in physiological range (b, d). BAC benzalkonium chloride, MGHS macrogolglycerol hydroxystearate 40
Histology
Regarding the corneal microstructure at the end of both long-term studies over 3 days (Fig. 7) or 5 days (Fig. 8), histological findings confirmed microscopic data for all test substances. Formulations A and B and CT show equal results after 3 days as well as after exposure and observation period extended to 5 days with complete healing of the epithelial layer, dense stroma, and regularly arranged keratocytes. In both studies, Descemet’s membrane and the endothelial layer were intact without any structural damage.
Histologic evaluation after 3 days drip application of the eye drugs A, B, C, and X and controls. Scale bar 100 µm. Formulations A and B and CT show complete healing of the epithelial layer, dense stroma, and regularly arranged keratocytes. Moreover, Descemet’s membrane and the endothelial layer were intact without any structural damage. For all corneas treated with substance C, re-epithelization with slight corneal edema was observed. Histology of the BAC-preserved latanoprost formulation X showed severe superficial corneal damage with complete epithelial cell loss, distinct local edema, and keratocyte loss with still intact Descemet and endothelial layers. The same result was seen on day 3 in the 0.02% BAC control group, even with detached Descemet’s membrane in one of three corneas. Hyaluronic acid-containing control CT shows perfectly and stably healed multilayered epithelium, intact stroma, Descemet, and endothelium. BAC benzalkonium chloride
Histologic evaluation after prolonged 5-day treatment with ophthalmic drugs A, B, and C and the control MGHS 5% (macrogolglycerol hydroxystearate 40) and hyaluronic acid CT. Scale bar 100 µm. Histological findings confirmed microscopic data for all test substances. Formulations A and B and CT show complete healing of the epithelial layer, dense stroma, and regularly arranged keratocytes. Moreover, Descemet’s membrane and the endothelial layer were intact without any structural damage. For corneas treated with substance C, the five corneas showed a total loss of epithelial cells, distinct stromal edema, severe loss of keratocytes, and a damaged endothelium detached from Descemet’s membrane. This formulation demonstrates very clearly the toxicological effects of eyedrops as a function of the observation period. Findings were similar for two out of five corneas treated with MGHS 5% (Fig. MGHS 5% 2) in contrast to the other three corneas of this group whose epithelium was histologically thin in places but closed (Fig. MGHS 5% 1). Hyaluronic acid-containing control CT in both studies shows perfectly and stably healed multilayered epithelium, intact stroma, Descemet, and endothelium
For all corneas treated with substance C, after 3 days, re-epithelization with slight corneal edema was observed. Once the observation period was extended to 5 days, all five corneas showed a total loss of epithelial cells, distinct stromal edema, severe loss of keratocytes, and a damaged endothelium detached from Descemet’s membrane (Fig. 8). This formulation demonstrates very clearly the toxicological effects of eyedrops as a function of the observation period.
Findings were similar for two out of five corneas treated with MGHS 5% (Fig. 8, “MGHS 5% 2”) in contrast to the other three corneas of this group whose epithelium was histologically thin in places but closed (Fig. 8, “MGHS 5% 1”).
After 3 days, histology of the BAC-preserved latanoprost formulation X showed severe superficial corneal damage with complete epithelial cell loss, distinct local edema, and keratocyte loss with still intact Descemet and endothelial layers (Fig. 7).
The same result was seen on day 3 in the 0.02% BAC control group, even with detached Descemet’s membrane in one of three corneas (Fig. 7).
Hyaluronic acid-containing control CT in both studies shows perfectly and stably healed multilayered epithelium, intact stroma, Descemet, and endothelium.
Discussion
The EVEIT system was used as an ex vivo model for this study and is suited for this type of research question [7,8,9,10,11,12,13] because it is able to document and quantify epithelial healing and toxic effects on the entire corneal structure in long-term experiments, mimicking a chronic use.
As described in the literature [8, 11, 14,15,16], the 3-day study highlights a strong toxic effect of the 0.02% BAC-preserved formulation and the control with BAC alone on the epithelium, which was also manifested in a damaged barrier function. Slight differences were also seen with PF eyedrops in the 3-day experiments, indicating that the safety profile may vary depending on the formulation of the excipients. The toxic effect could need a longer time period of testing to become apparent and significant.
The aim of the 5-day study was to compare the corneal toxicity of PF 0.005% latanoprost-containing eyedrops with different MGHS 40 concentrations (0%, 2.5%, and 5%), in order to highlight the impact of the overall PF formulation of glaucoma eyedrops on corneal integrity.
In fact, while extensive publications have already compared preserved to PF formulations, and in vivo and in vitro corneal toxicity of preserved glaucoma eyedrops has already been widely demonstrated [17,18,19], the attention to other excipients in the drug formulation has been less pronounced in the literature. Our experiment shows that certain excipients other than preservatives are linked to corneal toxicity, especially when in high concentration and associated with thickening agents. MGHS 40 at 5%, in particular, has already been suspected to cause corneal toxicity with tarsal infiltration of macrophages and inflammation [5, 11, 20]. Formulation C was well tolerated in an in vitro (3D HCE cell construct model) and in vivo (rabbit model) toxicity study, even though a slight increase in the number of apoptotic cells was observed [21]. However, recent in vitro reports document a significant concentration-dependent toxicity of MGHS 40, as well as a toxicity of the formulation C (containing a high concentration of MGHS 40) on HCE-2 cells [5]. Furthermore, an in vivo study showed that this same PF formulation causes a pro-inflammatory biological effect on accessory structures of the eye that is likely due to the eyedrop formula, especially the presence of solubilizers (MGHS 40 5%) [20]. These results were in accordance with those from our study, which showed that formulation C was the most cytotoxic amongst the tested PF eyedrops. Interestingly, however, and in contrast to our expectations from previous reports [11], it seemed to have an even greater impact on corneal integrity than isolated MGHS 40 5% did. This suggests that epithelial toxic effects are linked to an interaction with various excipients in the ophthalmic formulations. Tested formulation C contains 5% MGHS along with additional excipients such as macrogol 4000, carbomer, sorbitol, and EDTA. One possible explanation could be that the presence of thickening agents can increase the residence time on the ocular surface and play a significant role in enhancing MGHS toxicity. Further studies with the isolated individual components of ophthalmic formulations would be of interest to further confirm our results.
Other tested PF latanoprost formulations were found to have very good healing properties, even formulation B containing MGHS 40 2.5%. This is likely due to the much lower concentration and to the absence of thickening agents, further supporting our previous findings. In the first 3-day EVEIT experiment, as observed in the 24-h in vitro experiments by Pauly et al. [21], we highlighted slight differences between PF latanoprost formulations. To follow up with recent literature, we concluded that the exposure and monitoring durations were insufficient to reliably detect epithelial lesions. This was one reason that prompted the decision to investigate formulations over extended time frames in the second experiment. In comparison to the healing process over 3 days, this 5-day study gave homogeneous and unambiguous results for all tested formulations, demonstrating the importance of long-term eyedrop application monitoring, which is more in line with clinical practice, as topical antiglaucomatous therapy is administered over a long period of more than months or years.
The argument that the ex vivo system is too susceptible to toxicity, especially in the area where clinical, non-preserved, high-frequency artificial tear formulations are used, was discussed by Daull et al. [22]. This argumentation has been followed up by our group with a 1:1 experiment of animals versus EVEIT copying the Daull approach. We found higher susceptibility of the EVEIT system for ocular toxicity due to the lack of dilution effects and healing capacity of a living experimental animal body [12]. However, the long-term effects of drugs on the ocular surface are cumulative and lead to long-lasting pro-inflammatory reactions of the treated eye, which also affect the outcome of surgery [23].
There are major differences between latanoprost application in the EVEIT model and clinical administration of latanoprost in humans, including the posology and the duration of exposure. Additionally, the experiment tests the effect of topical glaucoma formulations on previously injured corneas. While patients with glaucoma do not have corneal abrasions in real life, they do have common ocular surface problems including dryness. The EVEIT system was designed to showcase long-term damage in a short period of time. A similar study on healthy corneas would be more interesting, but will need to be conducted on aged animals with long-term exposures for months. Another limitation is that the EVEIT model is a static one that lacks a lid-wiper effect and a tear drainage system, which are partially responsible for the clearance of topically applied medications. Models with lid-wipers were previously tested internally and were shown to result in worse outcomes due to the wiping of unstable and damaged epithelial structures. We therefore chose the EVEIT model that is not subject to mechanical damage. A tearing drainage system was mimicked by removing any excess fluid from the limbus, preventing long-term exposure of the limbus to any toxic substances. While such a model might have exaggerated the potential effect of thickening agents, it allowed us to showcase the toxicity of certain formulations in a short period of time. Highlighting the prolonged impact of formulation in real-life human studies is difficult and can take years, and no consensus has been found on preclinical eye toxicity models. The choice for ours was based on an extensive literature review and on reliability. The EVEIT model is a good predictor of toxicity as it allows a rapid evaluation of long-term exposure thanks to repeated administration and is totally in line with the 3R rule (replacement, reduction, refinement).
Conclusion
Our study shows that despite the removal of BAC from numerous eyedrop formulations to enhance their tolerability and safety, these formulations may still contain excipients that could potentially cause detrimental effects in the eye through different mechanisms. Even if PF formulations represent a remarkable progress, particular attention must be paid to the excipient formulation, e.g., the concentration of solubilizers and their combination with other components like thickening agents. Indeed, the association of MGHS 40 at a high concentration (5%) with thickening agents seems to be of concern for ocular surface health for a long-term glaucoma treatment.
References
European Glaucoma Society. European Glaucoma Society terminology and guidelines for glaucoma, 5th edition. Br J Ophthalmol. 2021;105:1–169.
Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22:730–5.
Konstas AG, Labbé A, Katsanos A, et al. The treatment of glaucoma using topical preservative-free agents: an evaluation of safety and tolerability. Expert Opin Drug Saf. 2021;20:453–66.
Hakkarainen JJ, Reinisalo M, Ragauskas S, Seppänen A, Kaja S, Kalesnykas G. Acute cytotoxic effects of marketed ophthalmic formulations on human corneal epithelial cells. Int J Pharm. 2016;511:73–8.
Smedowski A, Paterno JJ, Toropainen E, Sinha D, Wylegala E, Kaarniranta K. Excipients of preservative-free latanoprost induced inflammatory response and cytotoxicity in immortalized human HCE-2 corneal epithelial cells. J Biochem Pharmacol Res. 2014;2:175–84.
Balls M, Goldberg AM, Fentem JH, et al. The three Rs: the way forward: the report and recommendations of ECVAM Workshop 11. Altern Lab Anim. 1995;23:838–66.
Schrage N, Frentz M, Spoeler F. The Ex Vivo Eye Irritation Test (EVEIT) in evaluation of artificial tears: Purite-preserved versus unpreserved eye drops. Graefes Arch Clin Exp Ophthalmol. 2012;250:1333–40.
Frentz M, Goss M, Reim M, Schrage NF. Repeated exposure to benzalkonium chloride in the ex vivo eye irritation test (EVEIT): observation of isolated corneal damage and healing. Altern Lab Anim. 2008;36:25–32.
Spöler F, Kray O, Kray S, Panfil C, Schrage NF. The ex vivo eye irritation test as an alternative test method for serious eye damage/eye irritation. Altern Lab Anim. 2015;43:163–79.
Dutescu RM, Panfil C, Merkel OM, Schrage N. Semifluorinated alkanes as a liquid drug carrier system for topical ocular drug delivery. Eur J Pharm Biopharm. 2014;88:123–8.
Pinheiro R, Panfil C, Schrage N, Dutescu RM. The impact of glaucoma medications on corneal wound healing. J Glaucoma. 2016;25:122–7.
Dutescu RM, Uthoff D, Panfil C, Schrage N. High-frequency application of cationic agents containing lubricant eye drops causes cumulative corneal toxicity in an ex vivo eye irritation test model. J Ocul Pharmacol Ther. 2020;36:725–31.
Delafontaine M, Panfil C, Spöler F, et al. The ex vivo eye irritation test (EVEIT) model as a mean of improving venom ophthalmia understanding. Toxicon. 2018;150:253–60.
Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–23.
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–34.
Uematsu M, Kumagami T, Shimoda K, et al. Influence of alkyl chain length of benzalkonium chloride on acute corneal epithelial toxicity. Cornea. 2010;29:1296–301.
Liang H, Pauly A, Riancho L, Baudouin C, Brignole-Baudouin F. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95:869–75.
Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012;53:5154–60.
Mohamed YH, Uematsu M, Onizuka N, et al. Acute corneal toxicity of combined antiglaucoma topical eyedrops. Curr Eye Res. 2016;41:1326–30.
Trzeciecka A, Paterno JJ, Toropainen E, et al. Long-term topical application of preservative-free prostaglandin analogues evokes macrophage infiltration in the ocular adnexa. Eur J Pharmacol. 2016;788:12–20.
Pauly A, Roubeix C, Liang H, Brignole-Baudouin F, Baudouin C. In vitro and in vivo comparative toxicological study of a new preservative-free latanoprost formulation. Invest Ophthalmol Vis Sci. 2012;53:8172–80.
Daull P, Raymond E, Feraille L, Garrigue J-S. Safety and tolerability of overdosed artificial tears by abraded rabbit corneas. J Ocul Pharmacol Ther. 2018;34:670–6.
Okuda M, Mori S, Takano F, et al. Association of the prolonged use of anti-glaucoma medications with the surgical failure of ab interno microhook trabeculotomy. Acta Ophthalmol. 2022;100:e1209–15.
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fee were funded by Horus Pharma, Pharmaceutical Company (Nice, France).
Author Contributions
Laure Chauchat, Camille Guerin and Hayette Rebika developed the project’s concept. Marwan Sahyoun monitored communications. Claudia Panfil was responsible for data collection, analysis and drafting the paper, which was reviewed and revised by all authors. Claudia Panfil and Norbert Schrage are accountable for the data integrity. All authors approved the final paper.
Disclosures
Claudia Panfil and Norbert Schrage have nothing to disclose. Laure Chauchat, Camille Guerin, Hayetta Rebika and Marwan Sahyoun declare working for Horus Pharma at the time of completion of the manuscript.
Compliance with Ethics Guidelines
Approval by an ethics committee was not required for the experiments, as the eyes came from slaughtered animals. No live animal or human testing was involved in this work. No human tissues were involved and no patient data were used. Animal tissues involved were procured as waste product from the food industry which falls under the purview of the governmental German Veterinary Office.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Prior Presentation
The data included in this manuscript was presented as posters at the annual meeting of The Association for Research in Vision and Ophthalmology (ARVO), Denver, Colorado, USA, 2022 and New Orleans, Louisiana, USA, 2023.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Panfil, C., Chauchat, L., Guerin, C. et al. Impact of Latanoprost Antiglaucoma Eyedrops and Their Excipients on Toxicity and Healing Characteristics in the Ex Vivo Eye Irritation Test System. Ophthalmol Ther 12, 2641–2655 (2023). https://doi.org/10.1007/s40123-023-00769-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00769-y