Abstract
Purpose
To investigate multimodal retinal imaging characteristics including the retinal nerve fiber layer (RNFL) thickness in patients with RPGR-associated retinitis pigmentosa (RP).
Methods
This cross-sectional case–control study included 17 consecutive patients (median age, 21 years) with RPGR-associated RP who underwent retinal imaging including optical coherence tomography (OCT), short-wavelength fundus autofluorescence (AF) imaging, and RNFL scans centered on the optic disc. RNFL thickness was manually segmented and compared to clinical and imaging parameters including the transfoveal ellipsoid zone (EZ) width, the horizontal diameter of the macular hyperautofluorescent ring. RNFL thickness was compared to 17 age- and sex-matched controls.
Results
In patients with RPGR-associated RP, the EZ width (R2 = 0.65), the central hyperautofluorescent ring on AF images (R2 = 0.72), and visual acuity (R2 = 0.68) were negatively correlated with age. In comparison to controls, a significantly (p < 0.0001) increased global RNFL thickness was identified in RPGR-associated RP, which was, however, less pronounced in progressed disease as indicated by the EZ width or the diameter of the central hyperautofluorescent ring.
Conclusions
This study describes retinal characteristics in patients with RPGR-associated RP including a pronounced peripapillary RNFL thickness compared to healthy controls. These results contribute to the knowledge about imaging biomarkers in RP, which might be of interest for therapeutic approaches such as gene replacement therapies.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutations in the RPGR gene are the major cause of X-linked retinitis pigmentosa (RP). The retinal phenotype is characterized by pronounced alterations with blindness often within the third or fourth decade of life. Thus, it represents one of the most severe forms of RP in males [1,2,3,4,5,6,7,8,9]. Female carriers might also be affected to a variable degree, for instance, due to skewed X-inactivation, and RPGR variants may also be found in sporadic cases [9,10,11,12,13,14]. Therefore, it appears crucial to screen families with a provisional diagnosis of autosomal dominant or sporadic inheritance for variants in X-linked genes as well [15, 16].
With the development of novel treatment approaches for RPGR-associated RP, in particular gene replacement therapy, an explicit disease characterization including multimodal retinal imaging is increasingly important [17,18,19,20,21]. Previous natural history studies have shown an exponential decline of the ellipsoid zone (EZ) width and the hyperautofluorescent ring [22,23,24].
A phenomenon not fully understood is inner retinal thickening observed in RPGR-associated RP [25]. Explanations for this phenomenon have been brought forward including that it represents a neuronal-glial remodeling associated with photoreceptor stress or loss, which has been observed in rodent models of retinal degeneration [25,26,27]. The peripapillary retinal nerve fiber layer (RNFL) thickness represents a marker for inner retinal thickness, which can be measured by optical coherence tomography (OCT) imaging. Previously, an abnormal RNFL thinning and thickening has been reported in RP [28,29,30,31,32,33,34]. Even though the integrity of the inner layer is crucial for patients qualifying for e.g. gene replacement therapies, retinal implants, or optogenetic approaches, no study has specifically investigated RNFL alterations depended on the genetic disease cause and its impact is not comprehensively understood.
The present study provides a phenotypic characterization of patients with RPGR-associated RP, quantifies retinal layers with a focus on RNFL thickness, and compares RNFL alterations to healthy controls.
Methods
Patients
The subjects included in this cross-sectional case–control study were identified at the Department of Ophthalmology, University of Bonn, a dedicated clinic for retinal dystrophies. The study was in adherence to the declaration of Helsinki. Institutional review board approval (Ethikkommission, Medizinische Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn), and patients’ informed consent were obtained.
The clinical diagnosis of RP was based on the patient’s history, clinical examination, retinal imaging, and electrophysiologic assessment by full-field electroretinography (ERG). Genetic testing was performed as described previously [11, 35]. Exclusion criteria were any other pathology of the posterior pole unrelated to RP, any pathology affecting the ocular media like corneal opacities, cataract unusual for age or vitreous opacities, and highly unstable fixation, preventing adequate image acquisition. Furthermore, patients without RNFL imaging were excluded.
The RNFL thickness among the included patients was compared with a cohort of healthy controls (n = 17). These age-matched male subjects were unaffected by ophthalmic diseases and had a best-corrected visual acuity (BCVA) of 20/20 or better. Further cohort characteristics are provided in Supplementary Tables 1, 2.
Clinical examination, image acquisition, and analysis
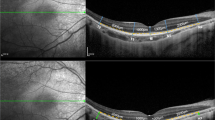
A complete ophthalmologic examination including BCVA testing, standardized slit-lamp examination, and dilated fundus examination was performed. Retinal imaging included fundus photography (Zeiss, Visucam, Oberkochen, Germany), wide-field pseudo-color- and AF fundus imaging (Optos PLC, Dunfermline, United Kingdom), spectral-domain optical coherence tomography (OCT) including RNFL ring scans (diameter of about 3.5–3.6 mm [36]) centered on the optic disc, and fundus autofluorescence (AF) imaging (both Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany).
Macular hyperautofluorescent rings identified by AF imaging, using a 488 nm laser excitation light, were measured manually by outlining horizontal borders with the Heidelberg Eye Explorer Software (HEYEX, Heidelberg Engineering). Furthermore, the software was used to measure the extent of the foveal EZ (based on central foveal scan) on OCT imaging. Exemplary AF and corresponding OCT images are provided in Supplementary Fig. 1.
Morphologic parameters and visual acuity of patients with RPGR-associated retinitis pigmentosa. Age was negatively correlated with visual acuity (a), the length of the visible foveal ellipsoid zone (b), and the horizontal diameter of the hyperautofluorescent ring using short-wavelength autofluorescence (d). The ellipsoid zone width (c) and the hyperautofluorescent ring (e) were both positively correlated with visual acuity
RNFL thickness of the circumpapillary area was measured between the inner border of the internal limiting membrane and the inner layer of the ganglion cell layer using the integrated RNFL measurement tool of the HEYEX. In a first step, the software performed the automated RNFL detection. Subsequently, a human grader checked the results for accuracy and corrected the automated segmentation manually if needed. The manual segmentations were re-checked by another independent grader. While the automated RNFL segmentation worked well for all healthy controls, it could not accurately segment the RNFL in all patients with retinitis pigmentosa and had to be corrected manually. Whole retinal thickness was peripapillary measured and obtained after manual segmentation of the internal limiting membrane and the retinal pigment epithelium/Bruch’s membrane complex. Non-RNFL layers were calculated by subtracting the RNFL from the whole retinal thickness. Mean values are reported globally as well as for six sectors relative to the disc-fovea axis (temporal, temporal superior, temporal inferior, nasal, nasal superior, nasal inferior).
Statistical analysis
Only the right eye of each patient was included. Statistical analysis was performed using GraphPad Prism v8.0 (GraphPad Software, La Jolla, CA, USA) and R [37]. The goodness-of-fit between the variables (BCVA, age, refraction, EZ, RNFL, refraction, hyperautofluorescent ring) was evaluated by (adjusted) R2 [38]. Multivariate regression with RNFL as dependent variable was performed accounting for potential interactions between the analyzed variables.
Results
Seventeen consecutive and unrelated male patients with RPGR-associated RP were included in this study. In all patients, the clinical diagnosis of RP was established based on patients’ history, characteristic RP fundus features including bone-spicule-like hyperpigmentation, vascular attenuation, optic disc pallor, and severely reduced or extinguished amplitudes on ERG examination. Median age at first symptoms was 8 years (interquartile range [IQR], 3-10 years). Median age at study examination and retinal imaging was 21 years (IQR, 16-39 years) and median BCVA (decimals) was 0.4 (IQR, 0.1–0.6). Cross-sectional analysis showed a negative correlation of visual acuity with age (slope= − 0.013 ± 0.002 decimal units BCVA/year; R2 = 0.68) (Fig. 1a).
On OCT scans recorded along the horizontal meridian, the width of the visible ellipsoid zone was negatively correlated with age (slope = − 45.37 ± 8.14 µm/year; R2 = 0.65) (Fig. 1b). By visual inspection, this effect seemed more pronounced in patients below the age of 20 years than in older patients. The width of the ellipsoid zone was positively (p < 0.001) correlated with visual acuity (slope= 2729.1 ± 593.5 µm per decimal unit BCVA; R2 = 0.56; p < 0.001) (Fig. 1c).
A symmetrical macular hyperautofluorescent ring using AF imaging was identified in 13 out of the 17 (76%) patients, the other 4 patients revealed either diffuse (n = 2) or patchy chorioretinal (n = 2) alterations on AF imaging. Patients with a hyperautofluorescent ring were younger (median age 21 vs. 54 years; p = 0.003) and had a better visual acuity (median BCVA 0.4 vs. 0.063; p = 0.0004) than patients without a ring. The horizontal measured diameter of the ring was negatively correlated with age (117.32 µm/year; R2 = 0.72; p < 0.001) which seemed by visual inspection more pronounced in patients below the age of 20 years (Fig. 1d) and positively correlated (p = 0.03) with visual acuity (slope = 4909 ± 1062 µm per decimal unit BCVA; R2 = 0.63; p < 0.001) (Fig. 1e).
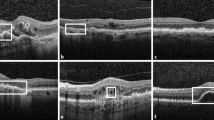
Measurement of mean global RNFL thickness in patients with RPGR-associated RP revealed significantly higher values compared to controls (128 µm vs. 96 µm; p < 0.0001). On a topographic level, the RNFL was significantly thickened in all sectors except for the nasal inferior sector (109.3 µm vs.107.8 µm) (Fig. 2a). The greatest thickness difference was seen in the temporal sector (136.9 µm vs.71.7 µm). In contrast, thinning of the outer retinal layers was observed in patients with RPGR-associated RP compared to controls. This resulted in significantly lower values of the whole retinal thickness in RPGR-associated RP globally (231 µm vs.309 µm; p < 0.0001) and in all different sectors (all, p < 0.0001) (Fig. 2b).
Representative peripapillary OCT scans of a healthy control and of a patient with RPGR-associated retinitis pigmentosa (top); sector-wise comparison of the retinal nerve fiber layer thickness (a) and whole retinal thickness (b) between RPGR-associated retinitis pigmentosa and controls. *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001
Subsequently, the impact of refractive error, age (both of which have been shown to affect RNFL thickness in normal eyes), EZ width, and the hyperautofluorescent ring diameter on RNFL thickness were analyzed. In univariate regression, age, the ellipsoid zone width and the hyperautofluorescent ring diameter were significantly associated with RNFL thickness (p = 0.014, p = 0.02, and p = 0.04, respectively) (Fig. 3b–d). However, no effect was observed for refractive error (p = 0.95) (Fig. 3a). Note that the EZ width and hyperautofluorescent ring diameter by themselves were highly correlated (Pearson’s correlation coefficient: 0.96).
To evaluate the individual contribution of each variable and account for possible interaction effects, multivariate linear regression was performed. After forward selection, age, EZ width and the interaction between those two were included in the final term (results provided in Supplementary Table 3). In summary, per year of age, the RNFL thickness decreased by 0.58 µm and per µm of EZ loss, the RNFL thickness decreased by 0.024 µm. Both factors alone, however, did not remain significant with the interaction term introduced into the model. Of note, with the limited number of RPGR-associated RP patients and the resulting moderate number of patients included in this analysis, the absence of statistical significance does not necessarily reflect the absence of a biological effect, but rather the limited power of the analysis. Finally, the interaction between age and ellipsoid zone width was significantly associated with RNFL thickness (p = 0.04): A patient who is 1 year older upon examination and has a 1 µm thinner EZ will have a 0.002 µm additional RNFL loss as compared to a patient where EZ remained unchanged over that year. Considering EZ loss as a surrogate for disease progression, these findings indicate that increased RNFL thickness was less pronounced in progressed disease. Together, the parameters included in this model explained 43.4% of the variability in RNFL thickness (i.e., adjusted R2 = 0.434).
Discussion
Our cross-sectional structural-functional approach of patients with RPGR-associated RP revealed that visual acuity, the EZ width, and the diameter of the hyperautofluorescent ring negatively correlated with age. The correlation between age and EZ width, with larger EZs in younger patients and a pronounced EZ decline in patients under the age of 20 years, is in accordance with previous reports identifying a faster rate of EZ decline in younger patients regardless of the underlying RPGR variant [22, 24].
A ring of increased autofluorescence was observed in 76% of our patients, which is slightly higher than observed in previous studies [23, 39]. Patients with a ring were younger and exhibited a better visual acuity than patients without rings, indicating more advanced disease in patients without rings, which might also explain different frequencies of this feature across studies. Patients under the age of 20 years revealed a more pronounced reduction of the horizontal ring diameter compared to older patients, indicating a decline in progression rate with age, also seen in other cohorts of RPGR-associated RP [23].
Quantification of RNFL thickness in RPGR-associated RP revealed a global and section-wise RNFL thickening, more prominent in the temporal than in the nasal sections. While RNFL thickening was independent of refraction error, an otherwise known effector of RNFL thickness, a lower RNFL thickness in older RP patients was observed. The calculated RNFL thickness decrease (per year of age) was even more pronounced as compared to healthy controls [40]. Moreover, RNFL thickening was less pronounced in advanced disease, measured by EZ width or the diameter of the hyperautofluorescent ring as indicated by multivariant regression analysis. While our study of RPGR-associated RP identified RNFL thickening in all patients, future studies may investigate whether and to what extent differences in RNFL thickness are present between different molecular disease causes. This may also explain the variability of RNFL thinning and thickening previously observed in RP [28,29,30,31,32,33,34]. However, as manual segmentation was necessary for all our RP patients, it cannot be excluded that segmentation artifacts or different OCT devices also contributed to the RNFL differences between previous studies.
Causes for RNFL thickening in RP but also in patients with other inherited retinal diseases such as choroideremia are currently not well understood and various potential explanations have been considered [32, 41]. These include microglial remodeling secondary to outer retinal atrophy or altered metabolic signaling, blood vessel architecture of the inner retina, or yet unknown factors [41,42,43,44,45]. However, with a lack of histological data, explanations of RNFL thickening remain, to some degree, speculative at present [41].
Phenotypic characteristics, as seen in this study, are of importance for patient selection and outcome measurements in interventional trials, but also for patient counseling on the future disease course. Individual characteristics may have different importance for particular aspects. For example, examining a ring of increased autofluorescence appears adequate evaluating patients with less advanced disease, while the EZ width may also reflect progressed disease. As the status of the inner layer is, for instance, crucial for patients qualifying for gene replacement therapies, retinal implants, optogenetic approaches, or induced-pluripotent stem cells, these results suggest at least no pronounced atrophy of the RNFL in RPGR-associated RP. However, as RNFL measurements are significantly thickened, the overall structural or functional integrity cannot be judged based on this study. Thus, further investigations of RNFL alterations appear important.
As RPGR-associated RP is a rare condition, we are aware of the potentially limited statistical power of this study due to the low number of included patients which is a common challenge in rare diseases. Moreover, the lack of longitudinal observations, the cross-sectional approach, and further factors influencing the RNFL represent limitations. In respect of the rapid developments of therapeutic approaches, additional studies appear prudent to validate these OCT-based findings in different cohorts of RPGR-associated RP but also across different molecular disease causes, and to test whether RNFL alterations can contribute to monitoring retinal dystrophies.
In conclusion, this study describes structural parameters and visual acuity of patients with RPGR-associated retinopathy and provides novel insights into RNFL alterations seen in RP patients. These findings may be of interest for future therapeutic approaches such as gene replacement therapies.
References
Verbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, Klaver CCW, Hoyng CB, Roepman R, Klevering BJ (2018) Non-syndromic retinitis pigmentosa. Prog Retin Eye Res 66:157–186. https://doi.org/10.1016/j.preteyeres.2018.03.005
Vervoort R, Lennon A, Bird AC, Tulloch B, Axton R, Miano MG, Meindl A, Meitinger T, Ciccodicola A, Wright AF (2000) Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet 25:462–466. https://doi.org/10.1038/78182
Talib M, van Schooneveld MJ, Thiadens AA, Fiocco M, Wijnholds J, Florijn RJ, Schalij-Delfos NE, van Genderen MM, Putter H, Cremers FPM, Dagnelie G, Ten Brink JB, Klaver CCW, van den Born LI, Hoyng CB, Bergen AA, Boon CJF (2019) Clinial and genetic characteristics of male patients with RPGR-associated retinal dystrophies: a long-term follow-up study. Retina 39:1186–1199. https://doi.org/10.1097/IAE.0000000000002125
Tee JJL, Yang Y, Kalitzeos A, Webster A, Bainbridge J, Weleber RG, Michaelides M (2018) Characterization of visual function, interocular variability and progression using static perimetry-derived metrics in RPGR-associated retinopathy. Invest Ophthalmol Vis Sci 59:2422–2436. https://doi.org/10.1167/iovs.17-23739
Bader I, Brandau O, Achatz H, Apfelstedt-Sylla E, Hergersberg M, Lorenz B, Wissinger B, Wittwer B, Rudolph G, Meindl A, Meitinger T (2003) X-linked retinitis pigmentosa: RPGR mutations in most families with definite X linkage and clustering of mutations in a short sequence stretch of exon ORF15. Invest Ophthalmol Vis Sci 44:1458–1463. https://doi.org/10.1167/iovs.02-0605
Pelletier V, Jambou M, Delphin N, Zinovieva E, Stum M, Gigarel N, Dollfus H, Hamel C, Toutain A, Dufier JL, Roche O, Munnich A, Bonnefont JP, Kaplan J, Rozet JM (2007) Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: genotype-phenotype correlations and impact on genetic counseling. Hum Mutat 28:81–91. https://doi.org/10.1002/humu.20417
Tee JJ, Smith AJ, Hardcastle AJ, Michaelides M (2016) RPGR-associated retinopathy: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol 100:1022–1027. https://doi.org/10.1136/bjophthalmol-2015-307698
Martinez-Fernandez De La Camara C, Nanda A, Salvetti AP, Fischer MD, MacLaren RE (2018) Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin Orphan Drugs 6:167–177. https://doi.org/10.1080/21678707.2018.1444476
Bird AC (1975) X-linked retinitis pigmentosa. Br J Ophthalmol 59:177–199. https://doi.org/10.1136/bjo.59.4.177
Nanda A, Salvetti AP, Clouston P, Downes SM, MacLaren RE (2018) Exploring the variable phenotypes of RPGR carrier females in assessing their potential for retinal gene therapy. Genes (Basel) 9(12):643. https://doi.org/10.3390/genes9120643
Birtel J, Gliem M, Mangold E, Muller PL, Holz FG, Neuhaus C, Lenzner S, Zahnleiter D, Betz C, Eisenberger T, Bolz HJ, Charbel Issa P (2018) Next-generation sequencing identifies unexpected genotype-phenotype correlations in patients with retinitis pigmentosa. PLoS ONE 13:e0207958. https://doi.org/10.1371/journal.pone.0207958
Comander J, Weigel-DiFranco C, Sandberg MA, Berson EL (2015) Visual function in carriers of X-linked retinitis pigmentosa. Ophthalmology 122:1899–1906. https://doi.org/10.1016/j.ophtha.2015.05.039
Grover S, Fishman GA, Anderson RJ, Lindeman M (2000) A longitudinal study of visual function in carriers of X-linked recessive retinitis pigmentosa. Ophthalmology 107:386–396. https://doi.org/10.1016/s0161-6420(99)00045-7
Wegscheider E, Preising MN, Lorenz B (2004) Fundus autofluorescence in carriers of X-linked recessive retinitis pigmentosa associated with mutations in RPGR, and correlation with electrophysiological and psychophysical data. Graefes Arch Clin Exp Ophthalmol 242:501–511. https://doi.org/10.1007/s00417-004-0891-1
Churchill JD, Bowne SJ, Sullivan LS, Lewis RA, Wheaton DK, Birch DG, Branham KE, Heckenlively JR, Daiger SP (2013) Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 54:1411–1416. https://doi.org/10.1167/iovs.12-11541
Birtel J, Gliem M, Oishi A, Muller PL, Herrmann P, Holz FG, Mangold E, Knapp M, Bolz HJ, Charbel Issa P (2019) Genetic testing in patients with retinitis pigmentosa: features of unsolved cases. Clin Exp Ophthalmol 47:779–786. https://doi.org/10.1111/ceo.13516
Cehajic Kapetanovic J, McClements ME, Martinez-Fernandez de la Camara C, MacLaren RE (2019) Molecular Strategies for RPGR Gene Therapy. Genes (Basel) 10(9):647. https://doi.org/10.3390/genes10090674
Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, Nanda A, Davies A, Wood LJ, Salvetti AP, Fischer MD, Aylward JW, Barnard AR, Jolly JK, Luo E, Lujan BJ, Ong T, Girach A, Black GCM, Gregori NZ, Davis JL, Rosa PR, Lotery AJ, Lam BL, Stanga PE, MacLaren RE (2020) Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med 26:354–359. https://doi.org/10.1038/s41591-020-0763-1
Scholl HP, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel JA (2016) Emerging therapies for inherited retinal degeneration. Sci Transl Med 8: 368rv366 https://doi.org/10.1126/scitranslmed.aaf2838
Birtel J, Gliem M, Holz FG, Herrmann P (2018) Imaging and molecular genetic diagnostics for the characterization of retinal dystrophies. Ophthalmologe 115:1021–1027. https://doi.org/10.1007/s00347-018-0779-9
Birtel J, Yusuf IH, Priglinger C, Rudolph G, Charbel Issa P (2021) Diagnosis of inherited retinal diseases. Klin Monbl Augenheilkd: 249–260. https://doi.org/10.1055/a-1388-7236
Tee JJL, Carroll J, Webster AR, Michaelides M (2017) Quantitative analysis of retinal structure using spectral-domain optical coherence tomography in RPGR-associated retinopathy. Am J Ophthalmol 178:18–26. https://doi.org/10.1016/j.ajo.2017.03.012
Tee JJL, Kalitzeos A, Webster AR, Peto T, Michaelides M (2018) Quantitative analysis of hyperautofluorescent rings to characterize the natural history and progression in Rpgr-associated retinopathy. Retina 38:2401–2414. https://doi.org/10.1097/IAE.0000000000001871
Tee JJL, Yang Y, Kalitzeos A, Webster A, Bainbridge J, Michaelides M (2019) Natural history study of retinal structure, progression, and symmetry using ellipzoid zone metrics in RPGR-associated retinopathy. Am J Ophthalmol 198:111–123. https://doi.org/10.1016/j.ajo.2018.10.003
Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA, Steinberg JD, Branham K, Othman M, Swaroop A, Jacobson SG (2007) Inner retinal abnormalities in X-linked retinitis pigmentosa with RPGR mutations. Invest Ophthalmol Vis Sci 48:4759–4765. https://doi.org/10.1167/iovs.07-0453
Marc RE, Jones BW, Watt CB, Strettoi E (2003) Neural remodeling in retinal degeneration. Prog Retin Eye Res 22:607–655. https://doi.org/10.1016/s1350-9462(03)00039-9
Jones BW, Marc RE (2005) Retinal remodeling during retinal degeneration. Exp Eye Res 81:123–137. https://doi.org/10.1016/j.exer.2005.03.006
Anastasakis A, Genead MA, McAnany JJ, Fishman GA (2012) Evaluation of retinal nerve fiber layer thickness in patients with retinitis pigmentosa using spectral-domain optical coherence tomography. Retina 32:358–363. https://doi.org/10.1097/IAE.0b013e31821a891a
Walia S, Fishman GA (2008) Retinal nerve fiber layer analysis in RP patients using Fourier-domain OCT. Invest Ophthalmol Vis Sci 49:3525–3528. https://doi.org/10.1167/iovs.08-1842
Walia S, Fishman GA, Edward DP, Lindeman M (2007) Retinal nerve fiber layer defects in RP patients. Invest Ophthalmol Vis Sci 48:4748–4752. https://doi.org/10.1167/iovs.07-0404
Oishi A, Ogino K, Nakagawa S, Makiyama Y, Kurimoto M, Otani A, Yoshimura N (2013) Longitudinal analysis of the peripapillary retinal nerve fiber layer thinning in patients with retinitis pigmentosa. Eye (Lond) 27:597–604. https://doi.org/10.1038/eye.2013.34
Oishi A, Otani A, Sasahara M, Kurimoto M, Nakamura H, Kojima H, Yoshimura N (2009) Retinal nerve fiber layer thickness in patients with retinitis pigmentosa. Eye (Lond) 23:561–566. https://doi.org/10.1038/eye.2008.63
Hwang YH, Kim SW, Kim YY, Na JH, Kim HK, Sohn YH (2012) Optic nerve head, retinal nerve fiber layer, and macular thickness measurements in young patients with retinitis pigmentosa. Curr Eye Res 37:914–920. https://doi.org/10.3109/02713683.2012.688163
Pasadhika S, Fishman GA, Allikmets R, Stone EM (2009) Peripapillary retinal nerve fiber layer thinning in patients with autosomal recessive cone-rod dystrophy. Am J Ophthalmol 148(260–265):e261. https://doi.org/10.1016/j.ajo.2009.03.001
Birtel J, Eisenberger T, Gliem M, Muller PL, Herrmann P, Betz C, Zahnleiter D, Neuhaus C, Lenzner S, Holz FG, Mangold E, Bolz HJ, Charbel Issa P (2018) Clinical and genetic characteristics of 251 consecutive patients with macular and cone/cone-rod dystrophy. Sci Rep 8:4824. https://doi.org/10.1038/s41598-018-22096-0
Wu H, de Boer JF, Chen TC (2011) Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma 20:470–476. https://doi.org/10.1097/IJG.0b013e3181f3eb64
R Development Core Team (2012) A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing
Linnet K (1990) Estimation of the linear relationship between the measurements of two methods with proportional errors. Stat Med 9:1463–1473. https://doi.org/10.1002/sim.4780091210
Nguyen XT, Talib M, van Schooneveld MJ, Brinks J, Ten Brink J, Florijn RJ, Wijnholds J, Verdijk RM, Bergen AA, Boon CJF (2020) RPGR-associated dystrophies: clinical, genetic, and histopathological features. Int J Mol Sci 21(3):835. https://doi.org/10.3390/ijms21030835
Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R (2007) Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology 114:921–926. https://doi.org/10.1016/j.ophtha.2007.01.023
Fu DJ, Xue K, Jolly JK, MacLaren RE (2019) A detailed in vivo analysis of the retinal nerve fibre layer in choroideremia. Acta Ophthalmol 97:e589–e600. https://doi.org/10.1111/aos.13973
Bojinova RI, Turksever C, Schotzau A, Valmaggia C, Schorderet DF, Todorova MG (2017) Reduced metabolic function and structural alterations in inherited retinal dystrophies: investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol 95:252–261. https://doi.org/10.1111/aos.13247
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC (2016) Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51:1–40. https://doi.org/10.1016/j.preteyeres.2015.06.003
Rattner A, Nathans J (2005) The genomic response to retinal disease and injury: evidence for endothelin signaling from photoreceptors to glia. J Neurosci 25:4540–4549. https://doi.org/10.1523/JNEUROSCI.0492-05.2005
Phillips MJ, Otteson DC, Sherry DM (2010) Progression of neuronal and synaptic remodeling in the rd10 mouse model of retinitis pigmentosa. J Comp Neurol 518:2071–2089. https://doi.org/10.1002/cne.22322
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Dr. Werner Jackstädt Foundation, Wuppertal, Germany (Grant S0134-10.22 to JB), the Novartis “EYEnovative” research award (to JB), the BONFOR Gerok Program by the University of Bonn (Grant 2019-1A-13 to KH), and the University Hospital Gießen and Marburg (Grant 15/2020MR to ML).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol and study documents were approved by the local ethics committee (Ethikkommission der Medizinischen Fakultät, Rheinische Friedrich-Wilhelms-Universität Bonn). This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
All authors declare no competing interests.
Disclaimer
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Theresa H. Birtel and Johannes Birtel contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Birtel, T.H., Birtel, J., Hess, K. et al. Analysis of imaging biomarkers and retinal nerve fiber layer thickness in RPGR-associated retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 259, 3597–3604 (2021). https://doi.org/10.1007/s00417-021-05233-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05233-w