Abstract
Purpose
To evaluate criteria driving retreatment with ranibizumab in Italian patients with myopic choroidal neovascularization (mCNV).
Methods
OLIMPIC was a 12-month, phase IIIb, open-label study. Patients with active mCNV were treated with ranibizumab 0.5 mg according to the European label. The study assessed local criteria in Italy driving retreatment decisions with ranibizumab; and the efficacy, safety, and tolerability of ranibizumab.
Results
The mean (standard deviation [SD]) age of treated patients (N = 200) was 61.8 (12.7) years; range 22–85 years. The multivariate regression model indicated that presence of active leakage (odds ratio [OR] 95% confidence interval [CI]: 11.30 [1.03–124.14]), presence of intraretinal fluid (OR [95%CI]: 28.21 [1.55–513.73]), and an improvement in best-corrected visual acuity (BCVA) from baseline < 10 letters (OR [95%CI]: 17.60 [1.39–222.75]) were the factors with the greatest effect on retreatment with ranibizumab. The mean (SD) BCVA gain from baseline to month 12 was 8.4 (12.8) letters (P < 0.0001). The mean (SD) number of injections was 2.41 (1.53); range 1–9. Ocular and non-ocular adverse events were reported in 41 (20.5%) and 30 (15.0%) patients, respectively.
Conclusions
Individualized treatment with ranibizumab was effective in improving BCVA in patients with mCNV over 12 months. Both anatomical and functional variables had significant effects on causing retreatment. There were no new safety findings.
Trial registration
www.ClinicalTrials.Gov (NCT No: NCT02034006)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathologic myopia (PM)—also known as high, degenerative, or malignant myopia [1]—is characterized by a refractive error of ≥ − 6 Diopter (D); axial length ≥ 26 mm; and degenerative changes involving the sclera, choroid, and retina [2, 3]. PM affects mainly the younger population (age < 50 years) [4,5,6], and is associated with a reduction in the quality of life (QoL) of the affected patients and is a socio-economic burden on society [7, 8]. Approximately 5–10% patients with PM develop myopic choroidal neovascularization (mCNV) [8], which is the most common vision-threatening complication of PM. The long-term prognosis of mCNV is poor if left untreated [5, 9,10,11].
Vascular endothelial growth factor (VEGF) is thought to have an important role in the pathogenesis of mCNV as it triggers angiogenesis and its levels are increased in eyes with mCNV [12,13,14]. Ranibizumab was the first anti-VEGF agent to be approved for the treatment of visual impairment secondary to mCNV based on the results from the RADIANCE [15] and REPAIR [16, 17] studies, which demonstrated superior visual and anatomical outcomes with ranibizumab. Following an initial injection, the current label of ranibizumab 0.5 mg for mCNV recommends monthly monitoring, and retreatment is determined by the physician based on disease activity, as assessed by visual acuity (VA) and/or anatomical parameters [18].
The OLIMPIC study (NCT02034006) [19] was designed to assess local criteria in Italy driving retreatment decisions with ranibizumab and to evaluate efficacy, safety, and tolerability of ranibizumab in Italian patients diagnosed with visual impairment due to mCNV.
Methods
Study design
OLIMPIC was a prospective, 12-month, phase IIIb, open-label, interventional, multicenter study in patients with visual impairment due to mCNV. The study was conducted from June 2014 to July 2016 across 33 centers in Italy (see Table, Online resource 1, which shows the list of study centers) and did not impose a fixed monthly visit scheme, used for registrative trial. The design used is very close to clinical practice.
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol (and all its amendments) was reviewed by the Independent Ethics Committee for each center. Patients provided written informed consent before entering the study.
Patients
Participants ≥ 18 years of age were included if they were diagnosed with active mCNV (as confirmed by the presence of high myopia with > − 6 D of spherical equivalence, posterior changes compatible with PM detected by fundus ophthalmoscopy and fundus photography, active leakage from CNV observed through fluorescein angiography (FA), and intra/subretinal fluid (IRF/SRF) observed using optical coherence tomography (OCT)) and had a best-corrected visual acuity (BCVA) > 24 and < 78 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (Snellen equivalent of approximately 20/32 to 20/320).
If both eyes were eligible, the eye with the worse VA at baseline was selected for treatment, except when medical reasons or local ethical requirements required selection of the eye with better VA.
Patients were excluded if they had an active infectious disease or intraocular inflammation in either eye at the time of enrolment; ocular disorders in the study eye requiring medical or surgical intervention or resulting in compromised VA; received pan-retinal or focal/grid laser photocoagulation with involvement of the macular area in the study eye at any time; or received intraocular treatment with any anti-VEGF, verteporfin photodynamic therapy, or any intraocular surgery or corticosteroid administration within 1 month before study entry; uncontrolled blood pressure; or a history of stroke or other medical conditions that could have influenced the study outcome. Women of childbearing potential not using effective methods of contraception, and pregnant or nursing women were also excluded.
Treatment
All eligible patients received a single initial intravitreal injection of ranibizumab 0.5 mg as per the approved label [18]. Mandatory monitoring visits were planned monthly for the first 3 months, and then at the 6 and 12 month after the first administration of ranibizumab, with a tolerance of 7 days. Optional visits could be possible outside of the pre-planned visits, but were not tracked in the electronic case record form. Further injections were administered if monitoring indicated the presence of disease activity, based on functional and/or anatomical features as per local clinical practice and as per approved label.
Objectives
The primary objective was to investigate the current criteria driving retreatment in Italian patients affected by mCNV and experiencing a relapse of the disease after the first administration of ranibizumab. Both anatomical (signs of lesion activity evaluated through clinical examination and/or OCT, and/or FA) and/or functional (reduction in BCVA) criteria were considered during the analysis.
The secondary objectives included evaluation of the mean change in BCVA at months 6 and 12 compared with baseline; the number of ranibizumab injections administered over the 12-month study period; time to relapse/retreatment; and safety and tolerability.
Assessments
Efficacy
Both functional and anatomical parameters were assessed locally by investigators or trained technicians at each site. Efficacy assessments were conducted before administration of ranibizumab on the day of treatment.
BCVA: BCVA was tested at each visit until month 12 using the ETDRS charts. BCVA measurements were taken in a sitting position at an initial test distance of 4 m using ETDRS charts. Refractive error was expressed in D.
OCT: Central subfield thickness (CSFT); central subfield volume (CSV); and presence of macular edema, IRF, cysts, and SRF were assessed using OCT at screening and months 2, 6, and 12.
FA: Evidence of CNV and presence of active leakage were assessed on FA at screening and months 2 and 6.
Clinically significant abnormalities assessed at fundus ophthalmoscopy and as per physician’s opinion (hemorrhage, pigment clumping, retinal pigment epithelium atrophy).
Safety
All adverse events (AEs) and serious adverse events (SAEs) were assessed over the entire 12-month study duration.
Statistical analysis
The planned sample size was 200 patients; assuming around 70% of patients would need retreatment, this meant that the primary endpoint could be evaluated in approximately 140 patients.
For the primary endpoint, a multivariate regression model was fitted considering the following covariates, using a stepwise procedure: presence of IRF (yes/no); presence of macular edema (yes/no); presence of cysts (yes/no); presence of active leakage (yes/no); presence of SRF (yes/no); clinically significant abnormalities, defined as any abnormal signal at the fundus examination, performed at each time point, assessed by means of a slit lamp and indirect stereo ophthalmoscope, as per clinical judgment (yes/no); change in macular volume versus previous visit; change in central retinal thickness versus previous visit; an improvement in BCVA < 5 letters (yes/no); an improvement in BCVA < 10 letters (yes/no); and change from baseline in BCVA classified as worsened (loss of ≥ 5 letters), stable (loss of ≥ 4 letters to gain of ≤ 4 letters), or improved (gain of ≥ 5 letters). A significance level of 0.3 was required to allow a variable into the model, and a significance level of 0.35 was required for a variable to remain in the model. Moreover, a univariate logistic regression model was applied to each of the aforementioned covariates.
A sensitivity analysis was then performed at month 2, which considered a patient as retreated if their first retreatment took place < 30 days after the month 2 assessment, in order to better evaluate the effect of each covariate. For all analyses, the independent variables were considered at the closest timepoint to the first retreatment. In case a value was not recorded at the scheduled assessment, the value was considered missing.
A Wilcoxon signed rank test was used to analyze changes in BCVA. A Wilcoxon/Mann-Whitney U test was used in case of non-normally distributed data for comparison of naïve versus treated patients. OCT and FA parameters were summarized using descriptive statistics. Time to retreatment in days was presented overall by Kaplan-Meier estimates and summarized by median, 25th and 75th percentiles, and their 95% confidence interval (CI). Efficacy was analyzed in the full analysis set (FAS) which included all patients who received at least one dose of ranibizumab.
Safety was analyzed in the safety analysis set which included all patients who received at least one dose of ranibizumab and had at least one post-baseline safety assessment. The safety results were summarized.
All analyses were carried out using SAS® for Windows release 9.4 (64-bit) or later (SAS Institute Inc., Cary, NC, USA).
Results
Of the 215 patients screened, 200 patients underwent a baseline visit and received one injection of ranibizumab 0.5 mg. All 200 patients were included in the FAS and safety set. Fourteen (7%) patients discontinued the study before the 12-month study visit. The reasons for discontinuation were consent withdrawn (n = 7), lost to follow-up (n = 5), death (n = 1), and pregnancy (n = 1, Fig. 1).
The mean (± standard deviation [SD]) age of the patients was 61.8 (12.7) years (range 22–85 years), 73.5% were female, and 99.5% patients were Caucasian. Bilateral PM was present in 79.5% patients and bilateral CNV in 13% patients. The most common CNV subtype observed was subfoveal CNV (61.0% patients). Median time from CNV diagnosis to informed consent was 0.89 (Interquartile range 0.10–14.74 months) (Table 1).
Of the 200 patients, 147 (73.5%) were anti-VEGF treatment-naїve at baseline. During the study, 70 (35%) patients were treated with ranibizumab 0.5 mg only once (defined as treated patients), and 130 (65%) patients were treated with ranibizumab more than once (defined as retreated patients). There were no differences in baseline characteristics between the treated and retreated cohorts or between the prior anti-VEGF treated and treatment-naïve cohorts (Table 1).
Efficacy
The multivariate regression model indicated presence of active leakage (OR [95%CI]: 11.30 [1.03–124.14]), presence of IRF (OR [95%CI]: 28.21 [1.55–513.73]), and an improvement in BCVA from baseline < 10 letters (OR [95%CI]: 17.60 [1.39–222.75]) to be the factors with the greatest effect on retreatment. The univariate model analysis indicated macular edema (P < 0.0001), presence of active leakage (P < 0.0001), presence of cysts (P < 0.0001), presence of IRF (P < 0.0001), presence of clinically significant abnormalities (P = 0.0103), and an improvement in BCVA of < 10 letters from baseline (P = 0.0114) to be the factors with a statistically significant association to retreatment (Fig. 2).
The sensitivity analysis at month 2 based on the multivariate regression model confirmed the presence of IRF and active leakage at month 2 to be the factors most likely to drive retreatment. The univariate model showed the presence of active leakage, IRF, SRF, cysts, and macular edema to be the factors strongly associated with retreatment.
BCVA outcomes
There was a notable gain in mean (SD) BCVA from baseline (54.66 [16.86]) to month 6 and to month 12 (7.51 [11.68] and 8.42 [12.81] ETDRS letters, respectively, both P < 0.0001, Fig. 3). The mean (SD) BCVA at baseline in patients who were anti-VEGF treatment-naïve or anti-VEGF-treated was 54.93 (17.00) and 53.89 (16.61) letters, respectively. The gain from baseline to month 12 or time of premature discontinuation was similar in patients who were prior anti-VEGF treatment-naïve or anti-VEGF-treated (8.32 [13.41] and 8.69 [11.12] ETDRS letters, respectively, both P < 0.0001, Fig. 3).
At month 12 or premature discontinuation, the proportion of patients gaining ≥ 5 and ≥ 10 ETDRS letters in the treated/retreated cohorts was 84.3/75.0% and 54.9/52.2%, respectively.
The mean (SD) refractive error remained stable in the study eye over the study duration, − 7.25 (6.09) D at baseline versus − 7.28 (6.51) D at month 12 or premature study discontinuation.
Anatomical outcomes
The foveal thickness decreased over time, and the mean (SD) change in CSFT from baseline (361.57 [92.16] μm) to months 6 and 12 or premature discontinuation was − 41.45 (77.88) and − 35.72 (95.28) μm, respectively (both P < 0.0001; Fig. 4). The mean (SD) CSFT at baseline was 335.98 (97.64) μm in the treated cohort and 374.97 (86.56) μm in the retreated cohort. The mean change in CSFT from baseline to month 12 was higher in retreated patients compared with treated patients (Fig. 4).The mean (SD) change in CSV from baseline (0.28 [0.07] mm3) at months 6 and 12 was − 0.02 (0.07) mm3 and − 0.02 (0.09) mm3, respectively (both P < 0.0001). The mean (SD) CSV at baseline was 0.27 (0.08) mm3 in the treated cohort and 0.29 (0.07) mm3 in the retreated cohort; the reduction in CSV at month 12 was similar in treated and retreated patients (− 0.02 [0.07] mm3 and − 0.01 [0.10] mm3, respectively).
The proportion of patients with macular edema, SRF, IRF, and cysts decreased from baseline to month 12 or premature discontinuation (see Fig, Online resource 2, which shows the proportion of patients with macular edema, SRF, IRF, and cysts during the study). The reduction in these anatomical parameters was more prominent in treated patients compared with retreated patients (see Fig, Online resource 2).
FA characteristics
At baseline, most patients underwent FA (187/200, 93.5%), and all, except 1 (1.56%) patient in treated cohort, had evidence of active CNV; this proportion decreased at month 2 (n = 121/180; 67.2%) and month 6 (n = 118/175; 67.4%) after ranibizumab treatment. The reduction from baseline at months 2 and 6 was observed in both treated and retreated cohorts.
At baseline, 182 of 187 patients undergoing FA (97.3%) showed active leakage. This proportion decreased at month 2 (n = 59/180; 32.8%) and month 6 (n = 44/175; 25.1%) after ranibizumab treatment (see Fig, Online resource 3, which shows the proportion of patients with active leakage from baseline to month 6).The reduction was observed in both treated and retreated cohorts at months 2 and 6 (see Fig, Online resource 3).
Treatment exposure
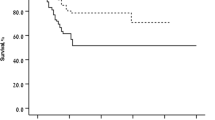
The mean (SD) number of injections in the FAS was 2.41 (1.53); range 1–9, the mean (SD) number of injections per retreated patient was 3.17 (1.40). Overall, 26.5%, 19.5%, and 8.5% of patients received two, three, or four injections, respectively, prior to month 12. Kaplan-Meier analysis of time to first retreatment showed that the median time for a patient to be free from retreatment was 3.15 months (95%CI: 2.33, 5.09 months, Fig. 5). This median time to relapse was similar in those who were anti-VEGF treatment-naïve or anti-VEGF-treated at baseline (3.19 and 3.15 months, respectively, Fig. 5).
Safety
Overall, at least one ocular or non-ocular AE was reported in 41 (20.5%) and 30 (15.0%) patients, respectively. Ocular SAEs were reported in two patients and non-ocular SAEs were reported in five patients (Table 2). Two AEs were suspected to be related to ranibizumab; both were ocular events (see Table, Online resource 4, which lists the drug-related AEs).
Two AEs, both non-ocular events (maternal exposure during delivery and spontaneous abortion) that occurred in one and the same patient, resulted in study discontinuation. Both events were suspected to be not related to ranibizumab. There were no cases of endophthalmitis. One death was reported during the study (due to cardiac arrest) and was considered by the investigator to be not related to treatment.
Discussion
The current European label for ranibizumab recommends individualized treatment based on disease activity, as assessed by VA and/or anatomical parameters, for patients with visual impairment due to mCNV [18]. In the OLIMPIC study, the results of the multivariate analysis showed two anatomical parameters (active leakage and IRF) and one VA parameter (improvement in BCVA from baseline < 10 letters) to be the factors most likely to drive retreatment. This finding was confirmed by the univariate model analysis results; of the 6 factors shown to drive retreatment, five were anatomical and the sixth was an improvement in BCVA from baseline < 10 letters. The sensitivity analyses performed at month 2 (both univariate and multivariate regression models) also showed anatomical parameters to be the main criteria driving retreatment.
The baseline characteristics of patients in this study were generally consistent with those reported previously in mCNV patients [15, 16, 20,21,22], in that most patients were female and the location of CNV was subfoveal in the majority of patients. However, patients in this study were older than those in the RADIANCE study (mean age ~ 62 years in OLIMPIC vs ~ 56 years in RADIANCE [15]). Ranibizumab treatment resulted in notable BCVA improvements at month 12 (+ 8.42 letters), which corroborated the benefits observed with ranibizumab in the RADIANCE and REPAIR studies (+ 14.4 letters and + 13.8 letters, respectively) [15, 17]. The nearly 1-line lower VA gain in OLIMPIC than that observed in RADIANCE could be related to the fact that patients enrolled in the OLIMPIC study were older, and a higher proportion had non-subfoveal lesions (61% in OLIMPIC vs ~ 70% in RADIANCE); also both treated and treatment-naïve patients were included. The VA improvements with ranibizumab have been shown to be maintained over the long term for up to 4 years in mCNV patients [23,24,25,26].
In patients with mCNV, it has been observed that VA improvements with ranibizumab occur irrespective of whether patients are treatment-naïve at baseline or not [27], whereas in other indications, better VA outcomes with ranibizumab treatment have been observed in treatment-naïve patients, which could be related to the difference in etiology of mCNV. Similarly, in the OLIMPIC study, there were no notable between-group differences, although the BCVA gain was numerically higher in anti-VEGF treatment-naïve patients.
In elderly patients, the inability to differentiate if CNV is related to pathologic myopia or to age-related macular degeneration is a known bias. In the pivotal phase 3 trials of ranibizumab and aflibercept in mCNV [15, 28], there were no upper limit in age for patient recruitment. In both the phase 3 studies, as well as the OLIMPIC study, all patients’ ≥ 18 years of age were eligible if affected with pathologic myopia and CNV. The mean (SD) age at baseline in the OLIMPIC study was 61.8 (12.7) years, range 22–85 years; in the ranibizumab groups in RADIANCE study was 54.0 (14.0) years, range 18–87 years (data on file) in group I and 56.1 (14.4) years, range 19–85 years (data on file) in group II; and in the aflibercept group in MYRROR study was 58.5 (13.17) years, range 23–87 years. As the inclusion criteria in the OLIMPIC study was similar to that in RADIANCE [15] and MYRROR [28], it has more or less the same bias with relation to age as that observed in the phase 3, registration studies that led to approval of ranibizumab and aflibercept for mCNV.
The anatomical outcomes corroborated with the BCVA improvements: there was a rapid reduction in CSFT at month 3 that gradually reduced further up to month 12. The change in CSFT was more prominent in retreated patients. The proportion of patients with macular edema, SRF, IRF, cysts, and active leakage also decreased over time. FA is considered the current standard of care for evaluating mCNV activity [23, 29, 30]. Most patients (93.5%) underwent FA analysis at screening in our study and all except one patient had evidence of mCNV. Ranibizumab treatment was associated with a reduction in the proportion of patients with active CNV and active leakage over time. In mCNV, the decision regarding retreatment needs a comprehensive approach to the disease. Although perception of metamorphopsia, VA changes, fundus examination, and OCT are often sufficient to indicate the need for retreatment, FA is an important tool to guide retreatment decisions for active mCNV [23, 29, 30].
Previous studies in patients with mCNV have shown that, on average, patients may require one to four anti-VEGF injections during the first year of treatment [15, 17, 27, 31,32,33]. Consistent with these reports, in our study, the mean number of ranibizumab injections was 2.41 over 12 months. After the mandatory first injection, 35% of patients did not require another injection, and 46% of patients required only one or two additional injections over the 12-month duration. The median treatment-free interval was 3.15 months, indicating that patients with mCNV may need less frequent monitoring compared with other ocular disorders.
The safety findings were consistent with the safety results from the pivotal trials and the known safety profile of ranibizumab [15,16,17,18]. The incidence of serious ocular and non-ocular AEs was low. There were no cases of endophthalmitis.
The limitations of the study are its open-label nature and lack of a placebo control. Furthermore, the study did not include classification of the staphyloma subtype. Although functional and anatomical outcomes were assessed in the study according to protocol, OCT and FA were not performed at each study visit, and FA was not assessed at the 12-month visit. Moreover, no central reading center was involved in the assessment of OCT and FA outcomes: despite procedures being described in the study protocol, a certain amount of variability in the assessment could be possible, in particular regarding potential overlapping criteria such as IRF, macular edema or intraretinal cysts, impacting the final anatomical outcomes and evaluation of the primary endpoint.
In conclusion, the OLIMPIC study confirmed that individualized treatment with ranibizumab is effective in improving and sustaining BCVA in patients with myopic CNV over 12 months. Many patients may only need one or two injections during the first year. Based on disease activity, the need for retreatment can be assessed by VA and/or anatomical parameters, but the latter were found to be the factors most likely to drive retreatment in this study.
References
Silva R (2012) Myopic maculopathy: a review. Ophthalmologica 228(4):197–213
Fredrick DR (2002) Myopia. BMJ 324(7347):1195–1199
Ohno-Matsui K (2016) Pathologic myopia. Asia Pac J Ophthalmol (Phila) 5(6):415–423
Miller DG, Singerman LJ (2006) Vision loss in younger patients: a review of choroidal neovascularization. Optom Vis Sci 83(5):316–325
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ (1996) Etiology of choroidal neovascularization in young patients. Ophthalmol 103(8):1241–1244
Wong YL, Saw SM (2016) Epidemiology of pathologic myopia in Asia and worldwide. Asia Pac J Ophthalmol (Phila) 5(6):394–402
Chiang PP-C, Fenwick E, Cheung CMG, Lamoureux EL (2014) Public health impact of pathologic myopia. In: Spaide RF, Ohno-Matsui K, Yannuzzi LA (eds) Pathologic myopia. Springer New York, New York, NY, pp 75–81
Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P (2014) Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 157(1):9–25 e12
Ohno-Matsui K, Yoshida T (2004) Myopic choroidal neovascularization: natural course and treatment. Curr Opin Ophthalmol 15(3):197–202
Miller DG, Singerman LJ (2001) Natural history of choroidal neovascularization in high myopia. Curr Opin Ophthalmol 12(3):222–224
Chan WM, Ohji M, Lai TY, Liu DT, Tano Y, Lam DS (2005) Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol 89(11):1522–1528
Wakabayashi T, Ikuno Y (2010) Choroidal filling delay in choroidal neovascularisation due to pathological myopia. Br J Ophthalmol 94(5):611–615
Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS (2006) Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 141(3):456–462
Wakabayashi T, Ikuno Y, Oshima Y, Hamasaki T, Nishida K (2013) Aqueous concentrations of vascular endothelial growth factor in eyes with high myopia with and without choroidal neovascularization. J Ophthalmol 2013:257381
Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, Wong TY, Silva R, Pilz S, Gekkieva M, Radiance Study Group (2014) RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmol 121(3):682–692 e682
Tufail A, Patel PJ, Sivaprasad S, Amoaku W, Browning AC, Cole M, Gale R, George S, Lotery AJ, Majid M, McKibbin M, Menon G, Yang Y, Andrews C, Brittain C, Osborne A (2013) Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye (Lond) 27(6):709–715
Tufail A, Narendran N, Patel PJ, Sivaprasad S, Amoaku W, Browning AC, Osoba O, Gale R, George S, Lotery AJ, Majid M, McKibbin M, Menon G, Andrews C, Brittain C, Osborne A, Yang Y (2013) Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmol 120(9):1944–1945 e1941
European Medicines Agency (2014) Summary of product characteristics. Lucentis 10 mg/ml solution for injection. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdf Accessed October 4, 2017
ClinicalTrials.gov (2017) A study of the criteria establishing the need for re-treatment with ranibizumab upon relapse in patients with visual impairment due to choroidal neovascularization secondary to pathologic myopia. (OLIMPIC). Available at: https://clinicaltrials.gov/ct2/show/NCT02034006 Accessed November 20, 2017
Wong TY, Foster PJ, Hee J, Ng TP, Tielsch JM, Chew SJ, Johnson GJ, Seah SK (2000) Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci 41(9):2486–2494
Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, Tokoro T, Mochizuki M (2010) Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmol 117(8):1595–1611 1611 e1591–1594
Hayashi K, Ohno-Matsui K, Yoshida T, Kobayashi K, Kojima A, Shimada N, Yasuzumi K, Futagami S, Tokoro T, Mochizuki M (2005) Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 243(1):13–19
Ladaique M, Dirani A, Ambresin A (2015) Long-term follow-up of choroidal neovascularization in pathological myopia treated with intravitreal ranibizumab. Klin Monatsbl Augenheilkd 232(4):542–547
Parravano M, Ricci F, Oddone F, Missiroli F, De Felici C, Varano M (2014) Long-term functional and morphologic retinal changes after ranibizumab and photodynamic therapy in myopic choroidal neovascularization. Retina 34(10):2053–2062
Lai TY, Luk FO, Lee GK, Lam DS (2012) Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye (Lond) 26(7):1004–1011
Ruiz-Moreno JM, Arias L, Montero JA, Carneiro A, Silva R (2013) Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol 97(11):1447–1450
Calvo-Gonzalez C, Reche-Frutos J, Donate J, Fernandez-Perez C, Garcia-Feijoo J (2011) Intravitreal ranibizumab for myopic choroidal neovascularization: factors predictive of visual outcome and need for retreatment. Am J Ophthalmol 151(3):529–534
Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, Stemper B, Asmus F, Zeitz O, Ishibashi T, Investigators M (2015) Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology 122(6):1220–1227
Introini U, Casalino G, Querques G, Gimeno AT, Scotti F, Bandello F (2012) Spectral-domain OCT in anti-VEGF treatment of myopic choroidal neovascularization. Eye (Lond) 26(7):976–982
Cheung CMG, Arnold JJ, Holz FG, Park KH, Lai TYY, Larsen M, Mitchell P, Ohno-Matsui K, Chen SJ, Wolf S, Wong TY (2017) Myopic choroidal neovascularization: review, guidance, and consensus statement on management. Ophthalmol 124(11):1690–1711
Wu TT, Kung YH (2014) Two-year outcome of intravitreal injections of ranibizumab for myopic choroidal neovascularization. J Ocul Pharmacol Ther 30(10):837–841
Willis J, Morse L, Vitale S, Parke DW II, Rich WL, Lum F, Cantrell RA (2017) Treatment patterns for myopic choroidal neovascularization in the United States: analysis of the IRIS registry. Ophthalmol 124(7):935–943
Kung YH, Wu TT, Huang YH (2014) One-year outcome of two different initial dosing regimens of intravitreal ranibizumab for myopic choroidal neovascularization. Acta Ophthalmol 92(8):e615–e620
Acknowledgements
The authors thank Lakshya Untwal and Lakshmi Venkatraman, (Scientific Services Practice—Product Lifecycle Services, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for the medical writing and editorial assistance towards the development of this article. The contribution of the IRCCS Fondazione Bietti in this paper was supported by the Italian Ministry of Health and by Fondazione Roma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Federico Ricci received consultation fees and travel grants from Alcon, Allergan, Bayer, Novartis and SIFI. Giovanni Staurenghi is a consultant/advisor for Heidelberg Engineering, Quantel Medical, Carl Zeiss Meditec, Alcon, Allergan, Bayer, Boehringer Ingelheim, Genentech, GSK, Novartis, Roche; has received grant support from Heidelberg Engineering, Optos, Optovue, Quantel Medical, Centervue; has received lecture fee from Alcon, Allergan, Bayer, GSK, Novartis, Roche; and patents/royalty from Ocular Instruments. Monica Varano received sponsorship from Allergan, Bayer, Novartis, and SIFI. Chiara Eandi received consultation fees and travel grants from Allergan, Bayer, Novartis, Optovue, and Thea. Marta Bartezaghi and Stefania Bassanini are employees of Novartis Farma SpA, Italy. Laura Colombo and Tommaso Lupieri Sinibaldi were employees of Novartis Farma SpA, Italy at the time of the study. This study was funded by Novartis Farma SpA, Italy.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 372 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ricci, F., Staurenghi, G., Varano, M. et al. OLIMPIC: a 12-month study on the criteria driving retreatment with ranibizumab in patients with visual impairment due to myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 257, 759–768 (2019). https://doi.org/10.1007/s00417-019-04248-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04248-8